ABOVE: Loss of neutrophils may lead to fever in cancer patients treated with radiation or chemotherapy. © ISTOCK.COM, JARUN011
One of the most common consequences of cytotoxic cancer treatments, such as chemotherapy and radiation, is the loss of a type of white blood cell called neutrophils—a phenomenon known as neutropenia. In some severe cases of neutropenia, patients develop a fever. Research published November 16 in Science Translational Medicine links this fever to mucus-degrading bacteria in the gut, specifically the commensal Akkermansia muciniphila. The study authors show that these microbes thin the mucus layer in mice, potentially exposing hosts to further bacterial infections—a finding that hints at possible ways to stave off treatment-related fevers in humans.
See “Gut Fungi Hamper Radiation Therapy in Mice with Cancer”
Previous work had hinted that infections originating in the gut can be a major source of bacterial infections in the bloodstream, and scientists have observed associations between changes in gut microbiota and neutropenic fever. To dig deeper into this link, a team led by scientists at the University of Texas MD Anderson Cancer Center analyzed the fecal samples from 119 patients undergoing stem cell transplantation, a procedure that is preceded by radiation and chemotherapy. They found that many of those who developed fever over the first days of neutropenia had an increased relative abundance of A. muciniphila and Bacteroides genera in their gut microbiomes, both of which are known to degrade mucin, a key component of the mucus layer.
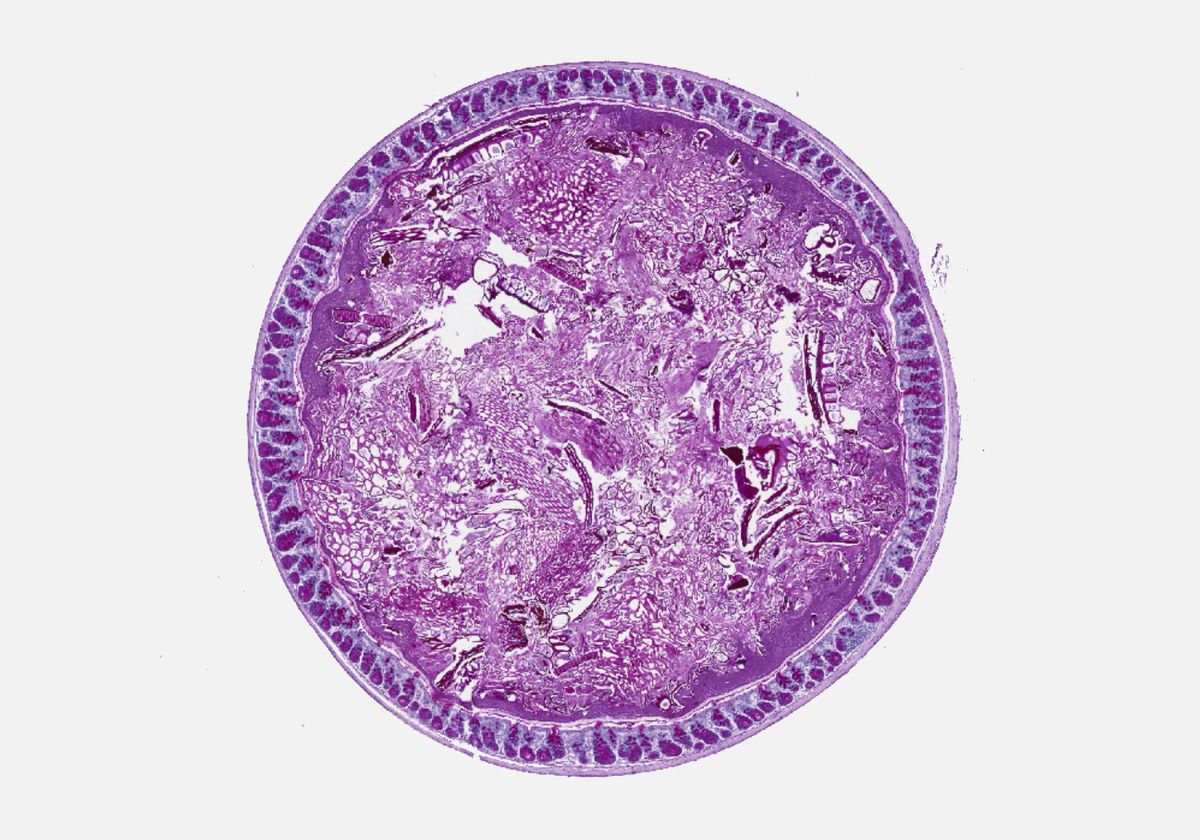
The team then determined whether radiation therapy or chemotherapy alters the microbiome composition of mice. Knowing that loss of appetite tends to follow cancer treatments, the team decided to launch a third experimental arm of the study when they found that the radiotherapy mice group started to eat less food. In this third group, they examined the microbiomes of healthy mice that were not exposed to any cytotoxic cancer treatment but were subjected to a calorie-restricted diet. A similar pattern emerged in all three experimental groups: levels of Akkermansia and, to a lesser degree, Bacteroides were increased six days after radiation or chemotherapy, or after one week of undergoing the restrictive diet, compared to control mice. These microbiome composition changes were accompanied by a significantly thinner mucus layer in the colon, revealed by histological analyses.
The mucus layer works as an intestinal barrier and, if it thins, it is easier for bacteria to make their way into the bloodstream and cause an infection, explains study coauthor and MD Anderson researcher Jennifer Karmouch. That is likely why some patients develop a fever, the team hypothesizes.
Karmouch and colleagues were particularly interested in finding out whether they could prevent Akkermansia from becoming more abundant and block the subsequent thinning of the colonic mucus layer. To do so, they gave mice one of two treatments. Some received the antibiotic azithromycin—which targets Akkermansia—while others were given propionate, a metabolite that the team found to be reduced in calorie-restricted mice’s colonic lumen. Furthermore, in an in vitro experiment, the team had observed that propionate prevents Akkermansia from consuming mucin.
Both treatments helped preserve the mucus layer in irradiated mice’s colons. While giving azithromycin to cancer patients is currently not recommended by the FDA, propionate may offer some hope, Karmouch says. Potential clinical use of the metabolite is currently limited due to poor absorbability, so Karmouch says she and her colleagues are currently trying to develop ways “to target it more directly to the colon.”
Despite the increased relative abundance of Akkermansia in the gut microbiomes of more than half of the patients who developed a neutropenic fever, the study shows that Akkermansia levels alone cannot predict if a patient with neutropenia will develop fever, as some cancer patients with fevers had low Akkermansia counts, and some without fevers had high levels of the bacteria. It is possible that the effect of these bacteria on the thinning of the mucus “might be strain-dependent,” says Karmouch—that is, there might be functional differences among A. muciniphila strains.
But there might be other explanations. Ami Bhatt, a microbiome scientist at Stanford University, says that the study shows a “very interesting association,” but it does not demonstrate “that Akkermansia is necessary or sufficient for” the thinning of the mucus, and there may be other mucin-degrading “organisms that can fill that niche.” Bhatt was not involved in this study but has previously collaborated with one of the coauthors and is on the scientific advisory board of Cantata Bio, an infectious disease-related startup.
Nonetheless, Bhatt adds, studies like this add evidence of how the gut microbiome can impact “the likelihood of potential pathobionts making it into the bloodstream and causing infections.”