Stay up to date on the latest science with Brush Up Summaries.
The immune system is a workhorse when it comes to protecting the body from foreign objects, including pathogenic microbes.1 Part of this protection is attributed to B cells, which produce billions of single species antibodies with unique antigen binding sites (ABSs).2 When an endogenous or exogenous antigen binds to an ABS, a series of events cause the foreign entity that the antigen is part of to be neutralized or destroyed.1 This brush-up summary reviews how researchers have adapted features of traditional antibodies to produce antibody therapeutics for disease treatments.
What Are Antibody Therapeutics?
Antibody therapeutics are a type of biological treatment that uses antibodies to target and neutralize or attack specific antigens while minimizing damage to healthy tissues. During neutralization, antibodies bind to their target, interfering with specific signaling pathways or blocking pathogens’ interactions with host cells.3 Direct attacks are facilitated by either antibody-dependent cell-mediated cytotoxic activity (ADCC) or complement-dependent cytotoxic activity (CDC).4 In ADCC and CDC, antibody binding enables cell lysis by recruiting immune cells. These targeted approaches minimize the risk of off-target effects that affect the integrity of normal cells, improving the safety profile of these therapies.4
What Are Monoclonal Antibodies?
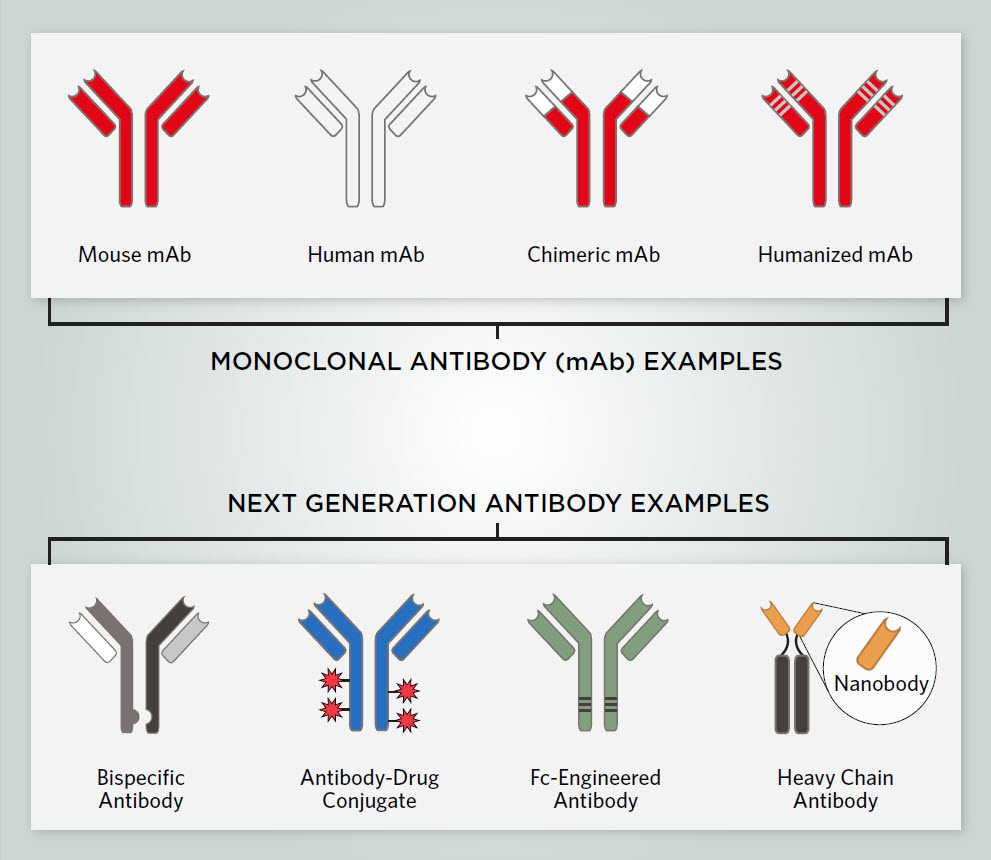
Monoclonal antibodies are highly specific for their antigen and have a well-characterized structure, making them ideal for use as antibody therapeutics.5 Each mAb, composed of two heavy and two light chains, has a tail (Fc) region with a constant sequence and two antigen binding (Fab) regions that contain the ABS formed from variable regions of the heavy and light chains.
Since researchers produced the first mAb therapeutic for treating non-Hodgkin’s lymphoma in 1997, mAbs have been one of the fastest growing drug classes.6 Therapeutic mAbs can be derived from humans, mouse-human chimeras, or humanized antibodies with mouse hypervariable regions grafted onto human antibodies. Fully human or humanized antibodies have the lowest risk of inducing an immune response that could destroy the mAb.7
Monoclonal Antibody Therapeutic Limitations
Despite their efficacy, mAbs have limitations and challenges that limit their effectiveness. One significant challenge is stability, as mAbs can unfold partially or completely denature, causing their aggregation.8 mAb aggregation can occur at any stage during the production process, compromising therapeutic quality and efficacy.9 Additionally, post-translational modifications of mAbs, such as glycosylation and oxidation, can lead to reduced efficacy and immunogenicity.10 Another major limitation is tissue penetrance. For example, in two mouse xenograft models bearing solid tumors from a human ovarian cancer line, only 20 percent of the mAbs targeted the tumor specific antigen, while the rest of the mAbs remained in the blood.11 These limitations have led researchers to produce more advanced antibody therapeutics.
What Are Next Generation Antibodies?
Scientists are developing next generation antibodies to overcome the above challenges, improve therapeutic antibodies’ efficacy and stability, and further minimize the risk of immune reactions. There are various types of next generation antibodies, including bispecific antibodies, antibody-drug conjugates, Fc-engineered antibodies, and nanobodies.7
Bispecific Antibodies
Bispecific antibodies (BsAbs) can bind to two different antigens simultaneously. BsAbs are often used in cancer immunotherapy, whereby they bind to a cytotoxic immune cell and a tumor specific antigen. Once in proximity, the immune cell can destroy the tumor cell.12
Antibody-Drug Conjugates
ADCs contain a cytotoxic drug attached to an mAb. When used as a cancer therapeutic, an ADC’s antibody component often targets antigens on the surface of cancer cells, delivering the attached drug which destroys the targeted cell.13
Fc-Engineered Antibodies
Fc-engineered mAbs have modifications at the Fc region, including amino acid substitutions and altered glycan binding. These changes can enhance cytotoxic effector functions such as ADCC and CDC.14
Nanobodies
Nanobodies are small antibody fragments derived from the variable regions of heavy-chain-only antibodies from camelids, a mammal family that includes camels, llamas, and alpacas.15 These antibodies are highly stable and can bind to target molecules with high specificity and affinity. Nanobodies can penetrate tissues and reach disease targets that are inaccessible to full-length antibodies.15

References
- B. Alberts et al., "B cells and antibodies," Mol Biol Cell, 4th edition, 2002.
2. B. Alberts et al., "The generation of antibody diversity," Mol Biol Cell, 4th edition, 2002.
3. J.J. Morales-Núñez et al. "Overview of neutralizing antibodies and their potential in COVID-19," Vaccines, 9(12):1376, 2021. doi: 10.3390/vaccines9121376
4. A. Natsume et al., "Improving effector functions of antibodies for cancer treatment: enhancing ADCC and CDC," Drug Des Devel Ther, 3:7-16, 2009. doi: 10.2147/DDDT.S4378
5. H.W. Schroeder, L. Cavacini, "Structure and function of immunoglobulins," J Allergy Clin Immunol, 125(2, Supplement 2):S41-S52, 2010. doi: 10.1016/j.jaci.2009.09.046
6. A. Forero, A.F. Lobuglio, "History of antibody therapy for non-Hodgkin’s lymphoma," Semin Oncol, 30(6 Suppl 17):1-5, 2003. doi: 10.1053/j.seminoncol.2003.10.002
7. R.M. Lu et al., "Development of therapeutic antibodies for the treatment of diseases," J Biomed Sci, 27(1):1, 2020. doi: 10.1186/s12929-019-0592-z
8. W. Li et al., "Antibody aggregation: insights from sequence and structure," Antibodies, 5(3):19, 2016. doi: 10.3390/antib5030019
9. M. Vázquez-Rey, D.A. Lang et al., "Aggregates in monoclonal antibody manufacturing processes," Biotechnology and Bioengineering, 108(7):1494-1508, 2011. doi: 10.1002/bit.23155
10. E. Edwards et al., "Strategies to control therapeutic antibody glycosylation during bioprocessing: synthesis and separation," Biotechnol Bioeng, 119(6):1343-58, 2022. doi: 10.1002/bit.28066
11. P. Holliger, P.J. Hudson, "Engineered antibody fragments and the rise of single domains," Nat Biotechnol, 1126-36, 2005. doi: 10.1038/nbt1142
12. R.E. Kontermann, "Dual targeting strategies with bispecific antibodies," mAbs, 4(2):182-97, 2012. doi: 10.4161/mabs.4.2.19000
13. Z. Fu et al., "Antibody drug conjugate: the “biological missile” for targeted cancer therapy," Signal Transduct Target Ther, 7(1):1-25, 2022. doi: 10.1038/s41392-022-00947-7
14. H.J. van der Horst et al., "Fc-engineered antibodies with enhanced Fc-effector function for the treatment of B-cell malignancies," Cancers, 12(10):3041, 2020. doi: 10.3390/cancers12103041
15. S. Sun et al., "Nanobody: A small antibody with big implications for tumor therapeutic strategy," Int J Nanomedicine, 16:2337-56, 2021. doi: 10.2147/IJN.S297631