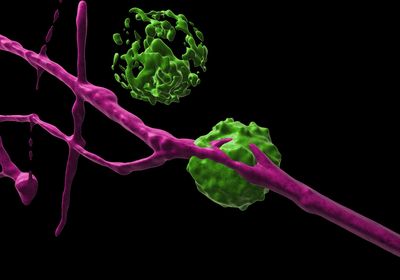
ABOVE: Research reveals three ways that dendritic cells (green) and nociceptor neurons (violet) communicate to fight off pathogens. PAVEL HANČ
Skin shields our bodies from the world’s dangers, but sometimes, with a nick or a bump, that barrier is breached. That’s when pain- and itch-sensing nociceptor neurons jump to action, transmitting threat signals to the central nervous system, while dendritic cells eliminate pathogens by secreting cytokines and coordinating local inflammation, while also playing a role in adaptive immunity.
But this work is not done in isolation: Dendritic cells (DCs) and nociceptors are entangled in a powerful partnership, and a new study published March 31 in Science describes three unique ways these intertwined cells communicate to fine-tune the fight against invaders.
Back in the early 2010s, clinicians observed that people with the autoimmune inflammatory skin condition psoriasis who also happen to have nerve damage don’t suffer from the condition’s hallmark skin lesions in the injured area. When the nerve heals, however, the lesions return. Lorena Riol-Blanco and Ulrich von Andrian, immunology researchers at Harvard University, were intrigued, and in 2014, their team discovered for the first time that DCs interact with nociceptors. They found that this relationship is necessary for an inflammatory response: DCs sit on nociceptor axons and require a signal from them to make the cytokine interleukin (IL)-23, which is the master driver of psoriasis skin inflammation.
The precise nature of this relationship, however, eluded them. “We didn’t know what’s the language that’s being spoken there,” von Andrian tells The Scientist. Answering “the question ’How exactly does it actually work?’ was a five-year process.”
Because the 2014 research used living mice, postdoctoral researcher Pavel Hanč, von Andrian, and their team, couldn’t be certain which of several different cell types were involved in nociceptor-to-DC communication. To narrow it down, the researchers first wanted to determine whether nociceptors spoke directly to DCs, or whether there was an unknown third cell that relayed the message. They set up coculture system with dorsal root ganglia neurons (including nociceptors) and bone marrow dendritic cells. The team then treated the cultured cells with a compound that causes an inflammatory psoriasis-like condition in mice and found that DCs produce more cytokines in the presence of the neurons—no other cell type was necessary.
Then, they examined what happened when the neurons and the dendritic cells shared the same culture medium but were separated by a filter that prevented them from touching. They found that the DCs “would actively try to squeeze themselves through these filter holes to find the neurons,” says von Andrian. The researchers reasoned, he adds, that there had to be some sort of “find me” signal coming from the nociceptor. So they used multiplexed assays to identify several known chemoattractants in nociceptor-conditioned medium, and when they depleted one of the most abundant, a chemokine called CCL2, DC migration towards the nociceptor-conditioned medium significantly decreased. The nociceptors released CCL2 in greater amounts when stimulated (in vivo, this stimulation would feel like pain or an itch). Nociceptors taken from mice genetically engineered to lack CCL2 did not attract DCs as well as those from wild-type mice.
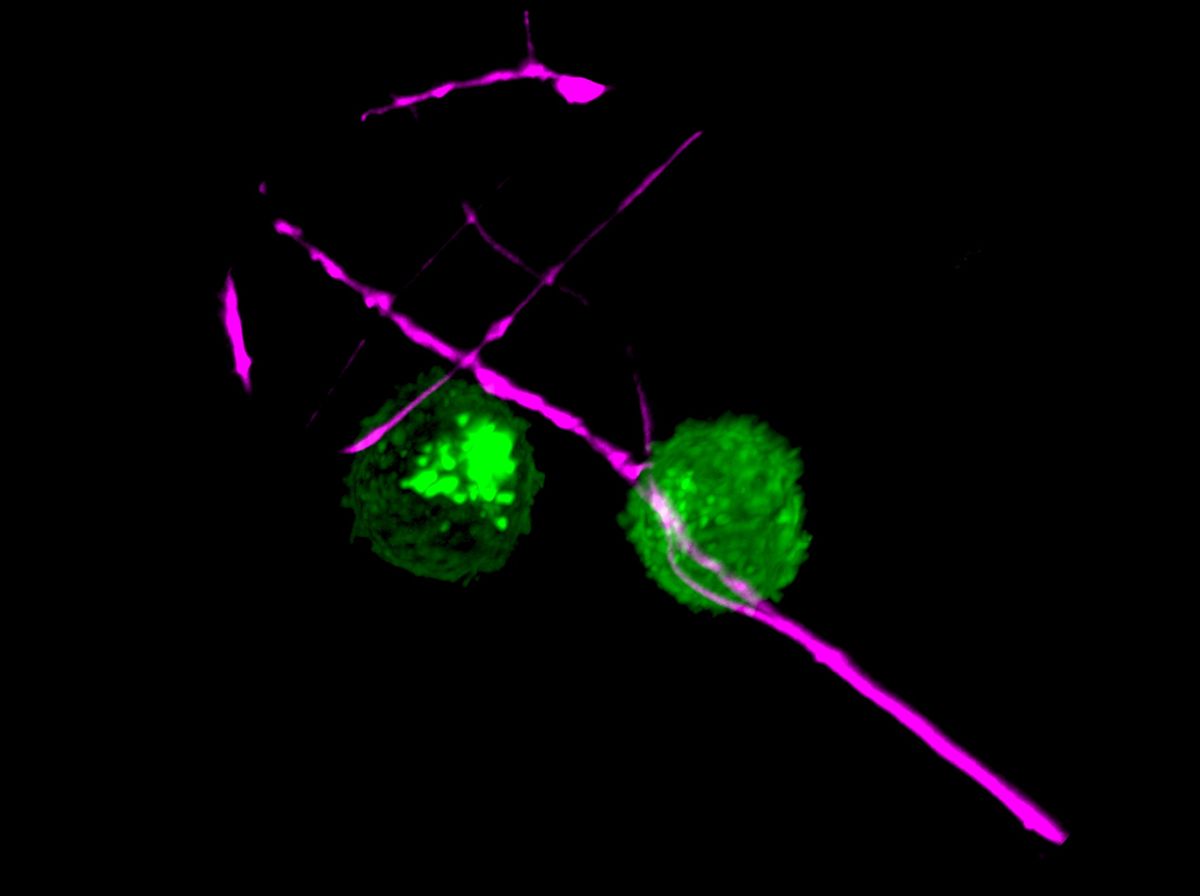
“The concept of neurons calling and keeping the DCs in place through CCL2 release is well-done and new, so that is very exciting,” says Caroline Sokol, a neuroimmunologist at Massachusetts General Hospital who didn’t work on the study.
The team also analyzed the transcriptome of DCs in the presence or absence of nociceptors and found that 983 genes had altered expression patterns between the two conditions. They found that a neuropeptide called CGRP released by the nociceptor neurons induces a transcriptional program responsible for most of the gene expression changes in DCs that allow them to fight pathogens. One of the most important changes is that CGRP triggers the DCs to produce pro-IL-1beta, which is the biologically inactive precursor of an important cytokine that the DC stockpiles for use in inflammation and other cellular activities.
“[Dendritic cells are] sort of a sentinel guard sitting in our barrier tissues on the lookout for any invading pathogens,” says von Andrian. “So, we interpret that CGRP signal coming from the nociceptors as a kind of ‘get ready’ signal” that primes DCs so they’re ready to be activated, he explains.
Using calcium imaging, the team found a third way that DCs and nociceptors communicate. They knew that the cells had to physically touch each other for DCs to produce cytokines. To see whether the cells communicated via this touch, they first treated the nociceptors in contact with DCs with capsaicin (the fiery component of chili peppers that activates nociceptors to induce the sensation of pain). This led to a rapid increase in intracellular calcium in both the neuron and abutting DC; this did not occur when DCs alone were treated with capsaicin. The neuron’s action potential, the team determined, extended to the DC in contact with the neuron, opening channels in the DC that allow calcium to stream into the cell, causing temporary membrane depolarization. When accompanied by a microbial immune stimulus directly on the DC, this increased calcium inside the cell has downstream effects that lead to an enhanced cytokine response. So, essentially, this third signal is “go,” says von Andrian.
Sokol says “They did a really beautiful job here, and this is begging other investigators to look at the same kind of output but with truly diverse real-world DC subsets that can respond to a whole host of different neuropeptides.”
This work could eventually lead to better drugs to treat inflammatory disease, says von Andrian, who has been involved in several companies (none having to do with nociceptors). But he also says he finds it fascinating to figure out the ways dendritic cells and nociceptors work together as a neuroimmune unit in both skin and mucosal surfaces. “They just use different means to survey our body for the presence of injury or infections,” he says. “So teleologically it makes perfect sense that these systems have evolved together and are in some sort of cahoots to do the best possible job in protecting us.”