ABOVE: © ISTOCK.COM, SEFA OZEL
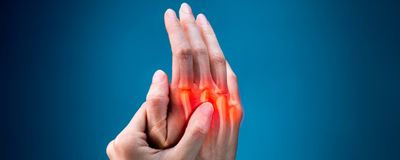
Rheumatoid arthritis (RA) is a debilitating autoimmune condition that affects millions of people across the globe (1). The ultimate cause of RA is largely mysterious. While researchers have long suspected that the microbiome influenced development of the disease, the specific microbe (or microbes) has eluded identification.
Now, in a recent Science Translational Medicine paper, researchers reported a strain of Subdoligranulum bacteria that may drive RA development (2). Some people at risk for the disease have antibodies against this bacteria, and Subdoligranulum activation of T cells was more prevalent in people with RA than in healthy controls. Perhaps even more intriguingly, mice given this bacterium developed a condition similar to human RA.
Identifying this bacterium was no simple task. First, the research team, a collaboration between scientists at the University of Colorado, Stanford University, and the Benaroya Research Institute, screened blood donated by people at risk for RA or with early-stage RA for RA-related autoantibodies.
Then researchers tested whether any of these autoantibodies also targeted human intestinal bacteria. They mixed the antibodies with bacteria from stool samples donated by healthy people and people with RA. They then sequenced the bacterial species to which the autoantibodies attached. These RA antibodies cross-reacted with many species of bacteria, largely from Lachnospiraceae or Ruminococcaceae, two closely related families.
To study these species in more detail, researchers cultured bacteria from the stool of an individual who had high levels of these two bacterial families present. Two types of Subdoligranulum bacteria, which they called isolates 1 and 7, emerged as potential candidates for driving RA development. Compared to isolate 1, isolate 7 was a more potent activator of T cells in blood from RA patients.
The reason I think this line of research is particularly exciting is that it could very well get at an actual beginning of the disease.
- Rabi Upadhyay, NYU Grossman School of Medicine
To find out if isolate 7 bacteria actually caused disease, scientists fed the bacteria to mice. Kristine Kuhn, a study coauthor and rheumatologist at the University of Colorado, said that she didn’t expect anything to happen when the team gave the mice the bacteria without another agent to disturb the immune system.
“We thought we were going to have to rev their immune systems up with an adjuvant or something,” said Kuhn. “Meagan [Chriswell, another author] was monitoring the mice, making sure that they had stable colonization. After a couple of weeks — I happened to be out of town — she called me up and she said, ‘Kristine, you'll never believe this, but the mice are getting swollen paws.” This is similar to the swollen hand and finger joints experienced by people with RA.
While other bacteria have previously been associated with human RA, Subdoligranulum is so far unique in its ability to cause RA-like symptoms in mice without the addition of another immune insult (3).
The similarities between the mice and human RA patients extended beyond what could be seen with the naked eye. “There were antibodies getting into the joints, much like we see in rheumatoid arthritis,” said Kuhn. “So, we started to profile the antibodies that were in the serum of the mice and we found that a lot of those antibodies targeted the same proteins that are targeted in rheumatoid arthritis.”
Rabi Upadhyay, a medical oncologist who studies the microbiome, immunity, and cancer at the NYU Grossman School of Medicine and was not involved in this work, said that while this study convincingly demonstrated that this species could produce an RA-like condition in mice, it may be too soon to pin all the blame on Subdoligranulum alone, since the study didn’t necessarily rule out other species.
“It's hard to know how big of a player this specific isolate is,” he said. “It could be the dominant player, and that's why they came across it first. But it could be that if they went back and did larger screens, that they would come up with more — that [Subdoligranulum] would be just one of many.”
In keeping with this, the researchers only found this Subdoligranulum strain in 16.7 percent of people at risk or with early-stage RA, indicating that this strain is likely not the sole driver of disease.
Nevertheless, Upadhyay said that it’s still an exciting study. Currently, there are no therapies that can prevent or cure the disease, and the immunosuppressant treatments that alleviate symptoms can have dangerous side effects.
“The reason I think this line of research is particularly exciting is that it could very well get at an actual beginning of the disease, so that we could design therapeutics to perturb the colonization of bacteria… to see whether you can prevent a percentage of disease prevalence by getting rid of colonization. And that would totally change the types of therapies that a rheumatologist could use.”
References
- Cross, M. et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 73, 1316–1322 (2014).
- Chriswell, M. E. et al. Clonal IgA and IgG autoantibodies from individuals at risk for rheumatoid arthritis identify an arthritogenic strain of Subdoligranulum. Science Translational Medicine 14, eabn5166 (2022).
- Scher, J. U. et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2, e01202 (2013).

This story was originally published on Drug Discovery News, the leading news magazine for scientists in pharma and biotech.