Roughly 2 million Americans will be diagnosed with cancer in 2023, and approximately 610,000 will die from the disease.1 A majority of those deaths will be due to advanced cancer progression, where tumor cells have spread to distant parts of the body and formed additional lesions.1 However, venturing through the depths of the body is no easy task—traveling cancer cells must survive harsh environments with scarce nutrients and low oxygen supplies.2
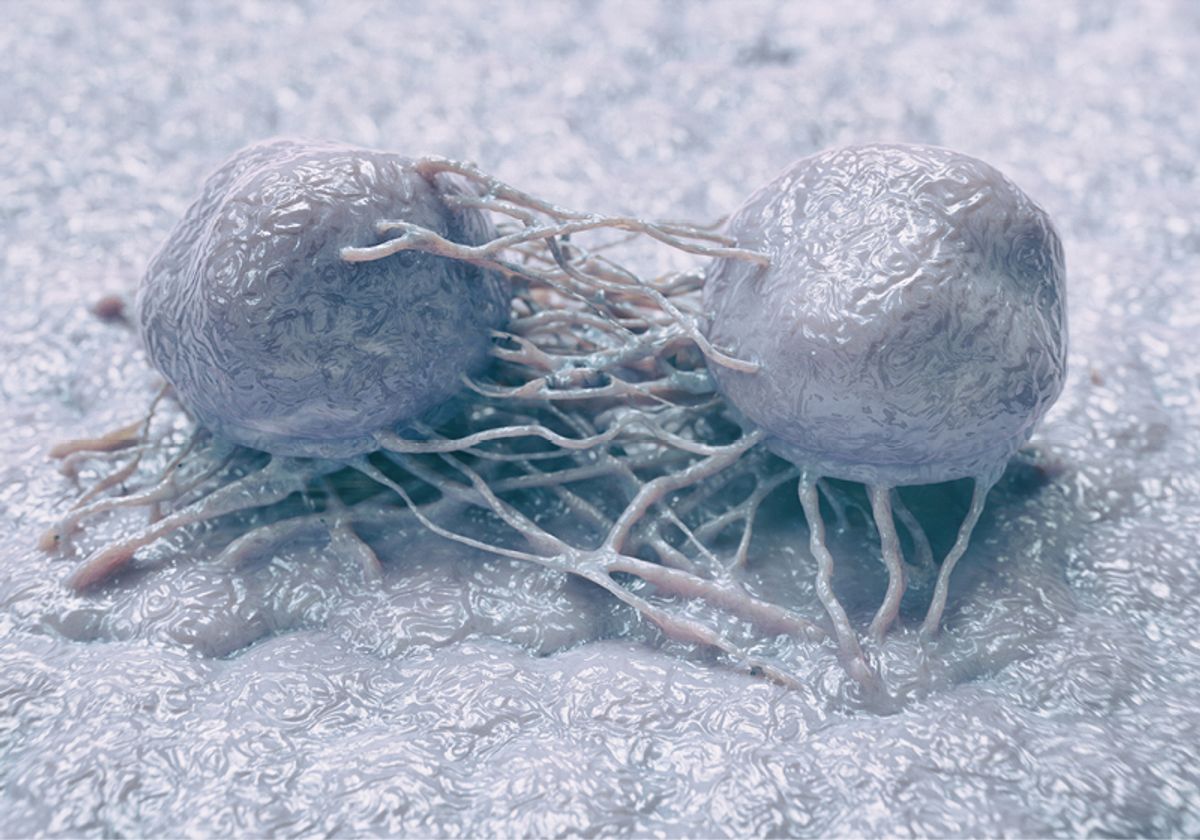
To access nutrients in challenging conditions, cancer cells use several mechanisms. One process, called entosis, is a form of cellular cannibalism where one cancer cell invades another.3 Once a tumor cell becomes internalized, it is degraded by the lysosome, which provides the outer cell with nutrients and other resources.3 A recent study published in iScience by Klaus Ebnet and his colleagues at the University of Münster provides insights into the molecular pathways important for entosis.
To study this process, the researchers used human breast cancer cells depleted of junctional adhesion molecule-A (JAM-A), a cell adhesion receptor that is localized at cell-cell junctions in polarized epithelial cells. Ebnet had previously shown that breast cancer cells lacking JAM-A are more motile and lose contact inhibition of locomotion (CIL)—a phenomenon whereby normal cells either stop moving or change their trajectories upon contact with each other. Researchers have previously associated loss of CIL with cancer cell dissemination during metastasis.4
“If JAM-A is lost due to downregulation, the cells are continuously migrating, and they have no more inhibition, so it's simply easier for the cell to engulf another cell or being invaded by another cell,” said Ebnet.
To see what happens when fluorescently-labeled cancer cells collide, the researchers cultured the cells on micropatterns—small, narrow strips coated with extracellular matrix proteins—to observe a few cells at a time migrating at high resolution. These narrow strips restricted the cells’ movement to one dimension, which ensured that they contacted each other.
If JAM-A is lost due to downregulation, the cells are continuously migrating and they have no more inhibition so it's simply easier for the cell to engulf another cell or being invaded by another cell.
- Klaus Ebnet, University of Münster.
“Doing it in such an approach, we can easily make conclusions of what type of process it is. It's easier to image, and it is easier to see what is happening with migration and invasion,” said Patricia Muller, a cancer biologist from Durham University, who was not involved in this study.
The researchers found that when the tumor cells were depleted of JAM-A and had lost CIL, they migrated into each other, forming cell-in-cell structures using an entotic process. Ebnet also observed that the inner cells were being degraded by enzymes in the lysosomes of the outer cells. Strikingly, the role of JAM-A in entosis was specific for tumor cells, as knockdown in noncancerous epithelial cells did not show entosis despite the loss of CIL.
Questions still remain about how entosis is regulated and the detailed molecular mechanisms that are at play. “I think one result of our study would be that people look more closely under regular microscopes [to see] if they can observe cell-in-cell structures and understand in more detail how entosis is regulated,” said Ebnet.
Overall, this work provides an avenue for studying how a lack of CIL and adhesion molecules such as JAM-A drive entosis, which may support cancer cells as they travel through the body.
References
- R.L. Siegel et al., “Cancer statistics, 2023,” CA Cancer J Clin, 73(1):17-48, 2023.
- B. Muz et al., “The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy,” Hypoxia, 83, 2015.
- S. Fais, M. Overholtzer, “Cell-in-cell phenomena in cancer,” Nat Rev Cancer, 18(12):758-66, 2018.
- B. Stramer, R. Mayor et al., “Mechanisms and in vivo functions of contact inhibition of locomotion,” Nat Rev Mol Cell Biol, 18(1):43-55, 2016.