Visualization is an integral component for studying cellular morphology and function. Unfortunately, living cells are translucent and largely composed of water, making them almost impossible to observe using traditional light microscopy. One solution for this problem is to stain cells with dyes, but while this process creates sufficient contrast for visualization, it typically kills the specimens. Fortunately, recent technological advances have made real-time, live-cell visualization much more broadly accessible, and researchers are taking advantage of this to see both cellular end-states and the transitory processes necessary to reach them.
Fluorescent labeling is one option for live-cell imaging. However, while this strategy allows researchers to observe living cells and visualize proteins or structures of interest, its invasive nature affects cell physiology. Furthermore, fluorescent labeling is not suited for long-term monitoring due to photobleaching.1 Phase contrast microscopy (PCM) offers a non-invasive alternative to dyes and labels. When light passes through translucent objects such as cells, it undergoes a phase shift relative to the illuminating (background) light. PCM captures these phase shifts, creates a phase shift gradient, and converts that into intensity variations for visualization. However, the technique is qualitative, not quantitative, meaning that the produced images are not ideal for making measurements.2
Introducing Quantitation
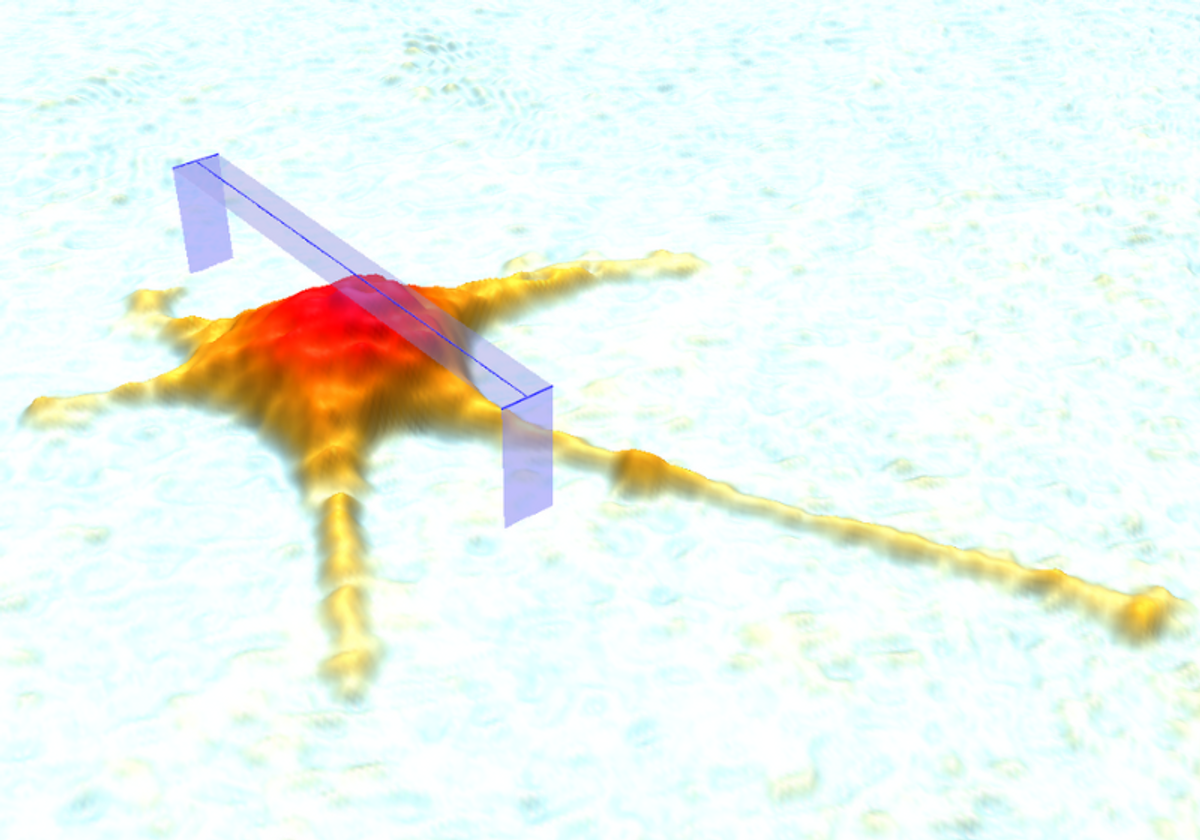
Quantitative phase imaging (QPI) is a label-free, non-invasive, and non-destructive visualization technique that can capture images in 2D (single plane), 3D (multi-plane), and 4D (multi-plane across time) with nanoscale sensitivity for morphology and dynamics.3 In QPI, light is refracted as it passes through a sample (the object beam). This altered light then merges with light that has not passed through the sample (the reference beam), creating an interference pattern that yields a hologram when it is detected by a sensor.2,4 Indeed, holographic microscopy—and more recently, digital holographic microscopy (DHM)—is the most commonly used form of QPI.
Researchers first used DHM to examine living cells around 2004-2005,5,6 and it quickly became popular owing to its non-invasiveness, very high resolution, capacity to image morphological features not visible via other techniques, potential for visualizing cellular dynamics, and ability to provide quantitative measurements.7 Digital holographic cytometry—the use of DHM for cellular research—can be automated, is cost efficient, is capable of high-throughputs, and, most critically, does not harm the cells. Researchers thus have used DHC to study a wide range of cell types, interactions, and processes.7
The HoloMonitor Solution

Long-term imaging of live cells needs to take place in a controlled environment to ensure cells are behaving in a physiologically relevant manner. It also needs to be non-invasive to avoid triggering cellular responses and automated to limit environmental perturbations. Ideally, it should also be compact in order to fit inside incubators, affordable, and easy to use. The HoloMonitor® live cell imaging system from PHI is a complete label-free, single cell resolution imaging solution. Comprised of the compact HoloMonitor cell culture microscope and the tailor-made and powerful App Suite software, the system naturally fits within typical standard cell incubators, making it perfect for cell culture monitoring, cell counting and quality control, and live cell experiments such as wound healing and motility assays.
References
- S. Aknoun et al., “Quantitative phase microscopy for non-invasive live cell population monitoring,” Sci Rep, 11:4409, 2021.
- Z. El-Schich et al., “Quantitative phase imaging for label-free analysis of cancer cells—focus on digital holographic microscopy,” Appl Sci, 8:1027, 2018.
- Y. Park et al., “Quantitative phase imaging in biomedicine,” Nature Photon, 12:578-89, 2018.
- L. Sternbæk et al., “Digital holographic cytometry: Macrophage uptake of nanoprobes,” Imaging and Microscopy, 1:21-23, 2019.
- P. Marquet et al., “Digital holographic microscopy: a noninvasive contrast imaging technique allowing quantitative visualization of living cells with subwavelength axial accuracy,” Opt Lett, 30(5):468-70, 2005.
- D. Carl et al., “Parameter-optimized digital holographic microscope for high-resolution living-cell analysis,” Appl Opt, 43(36):6536-44, 2004.
- K. Alm et al., “Cells and holograms – holograms and digital holographic microscopy as a tool to study the morphology of living cells,” in Holography—Basic Principles and Contemporary Applications, E. Mihaylova, ed., London: IntechOpen, 2013.