CRISPR-Cas9 revolutionized biology and healthcare. It hijacks the bacterial immune defense system to precisely edit a genome. But the method isn’t perfect. It can insert unwanted DNA sequences, delete segments entirely, and mistakenly mutate nontarget genes.
Research published in Science Advances today (July 1) reports that an alternative “soft CRISPR” called CRISPR-Nickase can efficiently and safely edit DNA.
CRISPR-Nickase leverages the fact that humans have two copies of each gene—one from each parent. Cas9, the molecular scissors that excise target genes, has two active sites that severs each copy. Nickase is a mutant of Cas9 with one site deactivated, so it just clips one.
Single cuts are useful when only one version of the gene is defective, a common occurrence in many genetic disorders. CRISPR-Nickase can remove the faulty copy, and the cell’s own DNA repair machinery can then copy the normal version in its place. This differs from traditional CRISPR-Cas9, where an experimentally introduced DNA template is required to repair the damage of a double-strand break—a process that can be error-prone, says study coauthor Ethan Bier, a developmental biologist at the University of California, San Diego.
Repairing nicks, however, is commonplace in cells. Each time a gene is copied or expressed, the cell nicks DNA to unwind it and repairs it in order to recondense. “This happens thousands and thousands of times a day,” says Bier, who has founded two companies based on CRISPR gene editing: Synbal Inc., which produces research animals, and Agragene, which develops pest control applications.
Because cells naturally repair nicks, the researchers hypothesized that CRISPR-Nickase could be an efficient way to edit single copies of a gene. In research published by other scientists, Nickase has shown promise in fruit fly sperm and eggs, which only carry one copy of each gene. This study shows that it also can work in somatic cells, which carry two.
Nobody expected that this would work so remarkably.
—Ethan Bier, University of California, San Diego
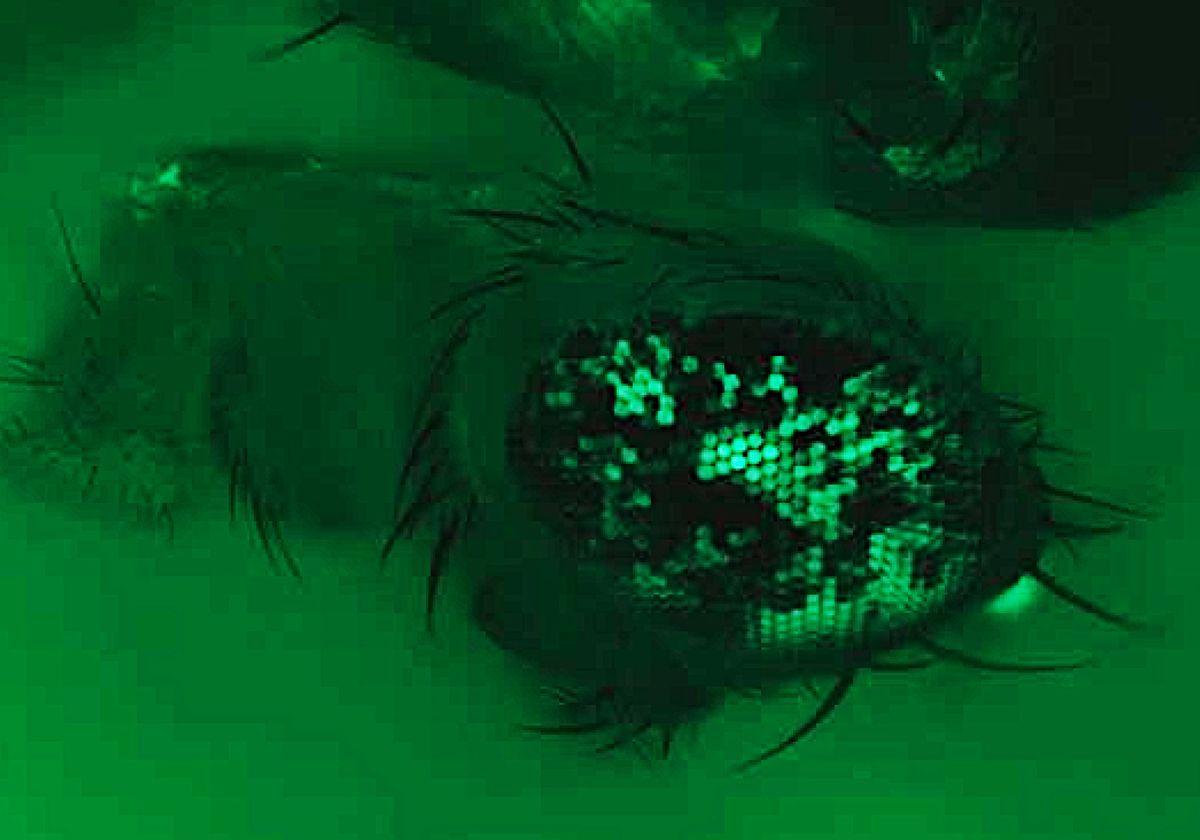
The team tested the new CRISPR technique by using it to modify eye color in fruit flies. The gene they targeted codes for red eyes when functional, but the lab-raised mutant flies they engineered had two defective copies that instead coded for white eye color.
Those copies differed in where the mutation occurred. Mom’s copy was functional at site one but mutated at site two, whereas dad’s copy was functional at site two and mutated at site one. The researchers devised CRISPR-Nickase to nick the DNA at the mutated paternal site 1 and replace it with the functional copy of that site from the maternal gene. The repaired paternal copy would then code for the production of red eye pigments, restoring normal eye color. Each eye was a mosaic of red and white, with red indicating successful gene editing.
The researchers tested the modified approach against traditional CRISPR-Cas9 and found that Nickase outperformed Cas9 by leaps and bounds. In CRISPR-Cas9-edited flies, an average of 20–30 percent of each fly’s eye turned red, but CRISPR-Nickase had a 50–70 percent success rate.
Study coauthor Annabel Guichard, a CRISPR researcher at the University of California, San Diego, says she was astonished when she first gazed into the edited flies’ piercing red eyes. “That was a fantastical, magical moment,” she says.
“I couldn’t believe it when [Guichard] showed it to me,” adds Bier. “Nobody expected that this would work so remarkably.”
In addition to being efficient, CRISPR-Nickase also carried less of a mutational risk compared to CRISPR-Cas9. Cas9 caused unintended mutations at the editing site 66 percent of the time. Nickase’s mutation rate was just 0.7 percent—a hundred-fold decrease.
This doesn’t include off-target mutations in nontargeted genes. According to Bier, CRISPR-Cas9 misses its target 1 to 2 percent of the time. These off-target mutations are particularly dangerous because they could accidentally break healthy genes and risk causing diseases like cancer. Nickase causes none.
See “New Technique Limits CRISPR-Cas9 Off-Target Mutations”
“It’s as clean as you can possibly get,” Bier says. “For an application down the road where you’d want to use it for gene therapy, that’s exactly what you want. No off-target and no on-target mutations.”
The minimal mutation rate in particular excites Vivian Vigliotti, who researches health services and public health needs at the Yale New Haven Health System and wasn’t involved with the study. “I think it’s wonderful,” says Vigliotti, who has published on healthcare applications of CRISPR. “That’s really what we’re after.”
Ben Ewen-Campen, a developmental biologist who studies CRISPR at Harvard Medical School, says that the advent of CRISPR-Cas9 was “like the Cambrian explosion of new techniques,” facilitating new discoveries and spawning potential applications. The efficiency with which CRISPR-Nickase works was the latest “surprising finding” to emerge from CRISPR-Cas9’s rapid evolution, he says.
See “CRISPR’s Adaptation to Genome Editing Earns Chemistry Nobel”
Because CRISPR-Nickase works in adult cells, the study authors say it may one day be helpful for treating genetic diseases—especially ones like Huntington’s or certain cancers where one bad copy can confer illness. If the other copy is functional, CRISPR-Nickase could replace the diseased version with the healthy one.
“It’s opening the doors for CRISPR and for the wonderfully hopeful aspects that we, as researchers, have been looking for,” Vigliotti says.
The technique needs to be validated in mammals before being tested in humans and perhaps making its way into the clinic. Bier says that it will almost certainly turn out to work better in flies than in mammals, because flies’ chromosomes align so that the template DNA is near the repair site, but mammalian cells aren’t so organized.
If the researchers can optimize the technique in mammals, though, the applications could be far-reaching. Molecular cargo carrying CRISPR-Nickase technology could potentially be delivered to hard-to-reach regions of the body like the liver and the retina.
“It’s hard to say [what the applications are] without knowing how efficient these systems will be,” Bier says. “But I think you want to reach into chunks of the body that are difficult to repair any other way.”