ABOVE: MODIFIED FROM © ISTOCK.COM, natrot, koto_feja
Around 2015, Katherine Chiappinelli was investigating the mechanism behind a group of drugs approved to treat blood cancers—and showing promise against other cancers—when she made a puzzling discovery. Earlier studies had suggested that these drugs, known as DNA methyltransferase inhibitors (DNMTis), worked by triggering the expression of tumor suppressor genes. But Chiappinelli, then a postdoctoral fellow in Stephen Baylin’s lab at Johns Hopkins University, also saw an upregulation in genes involved in innate immunity.
It turned out that the drugs, which reduce the number of methyl groups that attach to and block transcription of certain segments of DNA, had removed the methylation suppressing expression of sequences within the genome that closely resemble and likely originated from retroviruses. These so-called endogenous retroviruses could then be transcribed into virus-like RNA that incited an immune response in cultured ovarian cancer cells. “The cell acts like it has a virus,” Chiappinelli, now at The George Washington University, explains. This immune response could be harnessed to help fight malignancies. In a mouse model of cancer, the team found that DNMTis enhanced the effect of immunotherapy.
The finding was yet another example of the outsized effects that endogenous retroviruses and other “jumping genes”—fragments of DNA composed largely of repeating sequences that are capable of hopping around from one part of the genome to another—can have in diseases such as cancer. Transposable elements are part of the noncoding genome, the more than 98 percent of our DNA that doesn’t hold recipes for proteins, and they make up around half of our DNA. Decades of research point to their role in shaping the
evolution of genomes, and more recent work has revealed that these elements may also influence a wide range of diseases.
See “Can Viruses in the Genome Cause Disease?”
In particular, over the past decade or so, researchers such as Chiappinelli have been amassing a body of evidence that these elements can both help and hinder the growth of cancer. Studies show that transposable elements are expressed in multiple cancers and influence how the disease develops in a variety of ways. Buoyed by this growing body of research, some investigators have already begun devising new therapeutics specifically targeting transposable elements, with a handful of new companies dedicated to this goal. Chiappinelli consults for one such company called ROME Therapeutics.
The study of transposable elements is a “fast-growing” subfield of cancer research, says Benjamin Greenbaum, a computational biologist at Memorial Sloan Kettering Cancer Center. Researchers have been approaching this topic from multiple angles, he adds, and “it’s really just starting to all come together.”
A flood of discovery
In 1988, researchers at Johns Hopkins University were examining the genes of people with hemophilia—a heritable bleeding disorder characterized by blood that does not clot properly—when they spotted insertions of a transposable element, long-interspersed nuclear element-1 (LINE-1), in several sites along the gene for the coagulation protein factor VIII. At the time, transposable elements were largely seen as “junk DNA,” the term once given to the noncoding portion of the genome. But this study revealed that these elements can exert an effect: When a transposon landed in a gene, it could lead to disease. Researchers have since found more than 100 types of LINE-1 insertions that induce some sort of pathology.
LINE-1 sequences make up approximately one-fifth of the human genome. They belong to a subset of transposable elements known as retrotransposons—DNA fragments that can replicate themselves via an RNA intermediate and reinsert into the genome. LINE-1 also encodes a reverse transcriptase protein, which it uses to generate the RNA intermediate. (Other transposable elements, such as short interspersed nuclear elements, or SINEs, can co-opt this reverse transcriptase for replication.) Only around 100–150 of the hundreds of thousands of LINE-1 sequences in the human genome are capable of activation, and these are usually suppressed by methylation. But over the years, studies from several groups have revealed that in the genomes of cancer cells, this methylation is sometimes removed.
The big question is are these elements just associated with cancer progression, or are they helping to drive it?
—Katherine Chiappinelli, George Washington University
In 2012, researchers at Harvard Medical School reported that LINE-1 could insert copies of itself into the middle of genes that are commonly mutated in tumors, possibly contributing to cancer formation. Shortly after, other groups published reports corroborating these findings. For example, Kathleen Burns, a pathologist at the Dana-Farber Cancer Institute, and her team reported that the expression of proteins encoded by LINE-1 was a key feature of many human cancers, including colon, lung, and breast cancer. “It was a really exciting time in the field,” Burns says. “I think a lot of gratification came to all of us by using different tools and different approaches and having a few different labs converging on some of the same conclusions.”
In parallel, other groups, such as Chiappinelli’s, were gathering evidence for the importance of endogenous retroviruses. (Unlike LINE-1, endogenous retroviruses in humans can only generate viruslike molecules such as RNA; these elements are mere relics of previous jumping events and can no longer move around the genome.) Around the same time that Chiappinelli and her colleagues were investigating endogenous retroviruses and the immune response their transcripts provoked in cultured ovarian cancer cells, another group discovered something similar in another cancer type.
According to Chiappinelli, her advisor, Baylin, had been giving a talk about their findings when a researcher at the University of Toronto, Daniel De Carvalho, told Baylin that he had made similar observations in colorectal cancer cells. The two teams decided to cooperate by sharing their data. Baylin’s team went on to find exactly the same immune-activating effect of endogenous retroviruses in colorectal cancer, and the teams submitted their papers to the journal Cell at the same time. “I think that it was probably more easily accepted by the field because you had two different groups discovering the same thing,” Chiappinelli says.
Double-edged swords
Scientists are still working out the extent to which transposable elements may promote and, in other cases, hold back cancer growth. Broadly, researchers know that transposable elements are expressed at much higher levels in cancer cells than in normal cells, although it isn’t clear why. According to Chiappinelli, one hypothesis is that this expression occurs in the context of global dysregulation of the epigenetic landscape of tumor cells, where disruptions to methylation and other epigenetic processes are common. “The big question is are these elements just associated with cancer progression, or are they helping to drive it?” Chiappinelli says.
Transposable elements may, in rare instances, directly give rise to cancer-driving mutations. For example, in 2016, researchers reported a case where LINE-1 inserted itself into a tumor suppressor gene, initiating colorectal cancer. But it appears to be much more common for transposable elements to insert themselves into the cancer genome with no immediate cancer-boosting effect. In fact, some scientists suspect that in early cancer development, the insertion of these elements may be detrimental to a growing cancer cell.
In cells that have turned cancerous, expression of LINE-1 and other transposable elements typically increases. This increase in transposable element expression may inhibit cancer growth by triggering an immune response. “The moment a cancer cell allows LINE-1 elements to replicate, it turns into a big disaster,” says Andrei Gudkov, a professor of oncology at the Roswell Park Comprehensive Cancer Center. These elements can create new copies of themselves, which reinsert into the genome and produce mutations and chromosomal rearrangements. They can be expressed to make proteins such as reverse transcriptase, which can generate products triggering inflammation, and endonuclease, which cleaves DNA—changes that can harm a cell’s viability. “Cells with active LINE-1 elements behave as if they have a piece of uranium inside them. They are living under conditions of constant mutational pressure,” Gudkov explains. “It’s a massive genome and epigenome destabilizing factor.”
Yet while this type of activity might be detrimental to some cancer cells, the flip side of LINE-1 activity is that it creates a huge amount of genetic diversity, which may ultimately endow some cancerous cells with characteristics that enable them to thrive. People don’t typically succumb to primary tumors, Gudkov says; it’s the tumors that have evaded all lines of therapy and metastasized that are more dangerous. “We die from cancer because cancer is creative.” Gudkov and others posit that, within cancer cells, transposable elements generate genetic diversity in a manner akin to that seen throughout the course of evolution within populations—albeit in an accelerated manner.
To test whether targeting transposable elements could help treat cancer, Gudkov, Greenbaum, and others have examined the effect of giving cancer cells a so-called reverse transcriptase inhibitor that halts the activity of LINE-1. In animals, Gudkov’s team demonstrated that administering this inhibitor made cancer cells less capable of adapting to treatment and extended progression-free survival in mice. In a study published last summer, Greenbaum, Burns, and their colleagues found that a reverse transcriptase inhibitor slowed the progression of metastatic colorectal cancer in 9 of 32 patients with the disease.
Transposable elements in cancerIn healthy cells, transposable elements (TEs) are typically inactivated by methylation. But in cancer cells, these elements can become demethylated, enabling them to be expressed (1). Some transposable elements code for proteins such as endonuclease and reverse transcriptase (2). The activity of these proteins enables transposable elements to reinsert themselves into DNA, causing damage and mutations (3). TEs that are unable to reverse transcribe may still be translated into antigens, which are can subsequently be expressed on the cell surface (4). The expression of transposable elements can also activate the cell’s innate immune response in various ways, such as through DNA damage or via the presence of TE-derived RNA in the cytoplasm (5), an effect that may subsequently alter a cancer’s ability to spread. 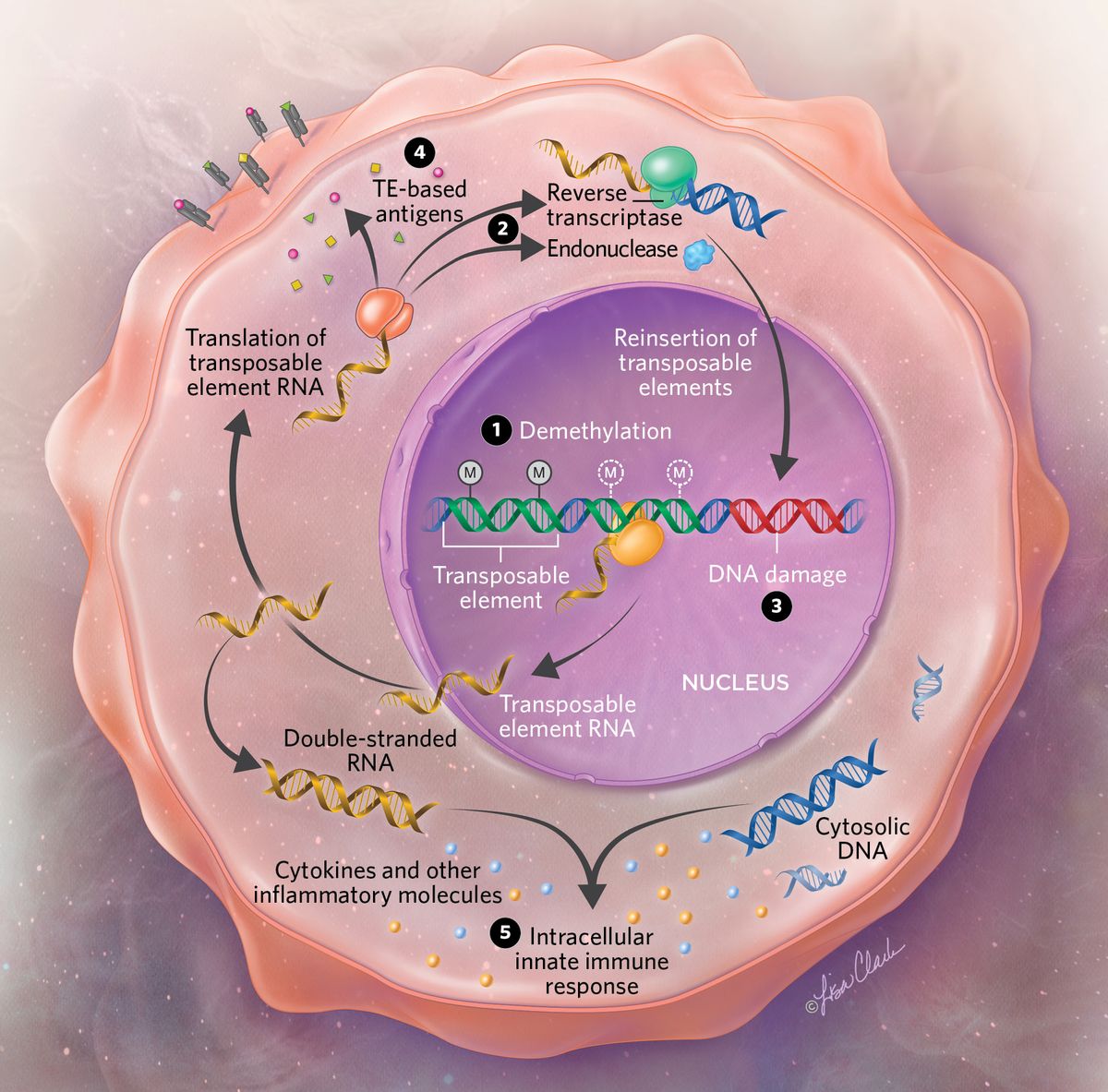 © Lisa Clark |
See “Cancer Researchers Use Evolution to Target Drug Resistance”
To the clinic
Researchers have already begun developing anticancer drugs targeting transposable elements. These elements have “definitely gotten the biotech and pharma interest over the last two or three years in a way that would have been quite shocking five to ten years ago,” says Greenbaum, who cofounded ROME Therapeutics to carry out this task. Gudkov, too, has founded a company, Genome Protection Inc, to develop such treatments. Some of this work has already spurred clinical trials. For example, Gudkov says that his team recently launched a study examining the effect of a reverse transcriptase inhibitor in small cell lung cancer patients.
Companies can choose from a variety of ways to target these elements. According to Nicolas Vabret, an immunologist and virologist at Mount Sinai’s Icahn School of Medicine who consulted for ROME on one occasion, these include using epigenetic drugs to induce elements to activate an immune response, as Chiappinelli unexpectedly discovered several years ago, or engineering CAR-T cells to express the RNA from transposable elements to enhance these cells’ immunotherapeutic activity.
Some researchers, including Burns, are also examining whether these elements might be useful for cancer diagnosis. If signs of changes in the epigenetic activation of these elements can be found in tissue samples of body fluids such as blood, they could be used as biomarkers of the disease, says Burns, who has consulted for ROME and other companies developing transposon-based cancer therapeutics.
Still, many open questions remain. To what degree do these elements contribute to cancer evolution or to the immune activation that might help fight cancers? At which specific location on the genome are these transposable elements being activated, and how does this differ across cancer types? Another key consideration, notes John Coffin, a molecular virologist at Tufts University and a shareholder and member of the scientific advisory board of ROME, is what these elements might be doing in healthy individuals. In a recent study, he and his colleagues discovered that endogenous retroviruses are expressed in multiple human tissues despite being thought to be largely silenced by methylation in healthy cells. “It’s important to have some idea of what the normal expression of these is, and in which tissue, because that’s a possible site of off-target effects of your therapy.”
All the same, the field of transposable elements is now overcoming earlier challenges as more researchers acknowledge that noncoding DNA is far from irrelevant, and as new technologies make it easier to study repetitive sequences. In particular, “we’re becoming more rigorous and building the right types of tools to approach the questions that we want to ask,” Burns says. “There’s been a complete paradigm shift in how we’re thinking about transposable elements and their possible functions in cancers. . . . There’s a lot of cool stuff on the horizon.”
Diana Kwon is a freelance science journalist based in Berlin, Germany.