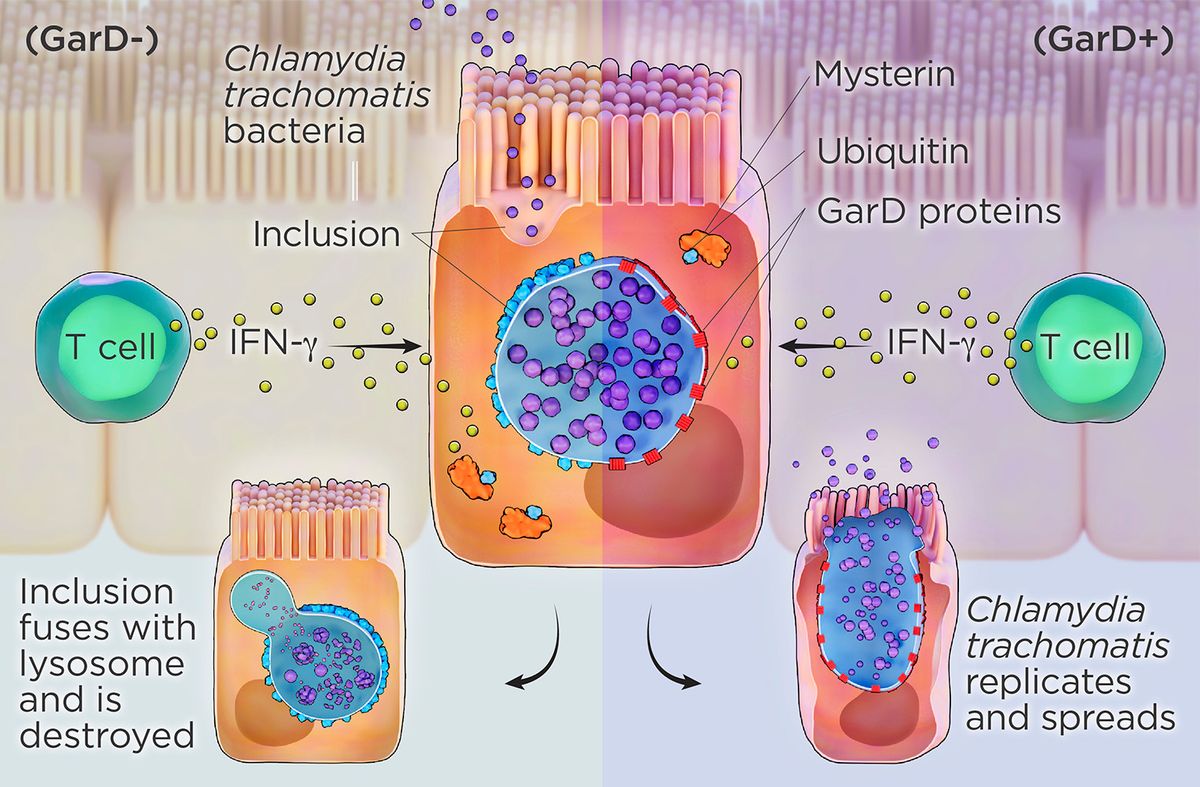
Chlamydia invades a host cell, forms a membrane-bound vacuole, or inclusion, and then modifies the protein composition of the structure’s membrane. If immune cells detect Chlamydia before it forms the inclusion, they trigger T cells to produce interferon-γ (IFN-γ), a powerful cytokine. IFN-γ activates the protein mysterin (also called RFN213), which attaches ubiquitin to the inclusion membrane, signaling the cell to destroy the inclusion’s contents by dumping them into a lysosome (left). C. trachomatis produces GarD, a protein that integrates into the inclusion membrane itself and somehow prevents mysterin from attaching ubiquitin, allowing the bacterium to evade immune destruction while continuing to multiply and eventually bursting from the cell (right).
Read the full story.