ABOVE: © ISTOCK.COM, Aldona
When you swallow a pill, only a fraction of the drug ends up where it’s needed. Active compounds diffuse across the intestinal wall and are diluted in rivers of blood, aimlessly drifting with the currents. For more precise delivery, scientists are recruiting motile, single-celled organisms as vehicles that transport drugs to specific sites in the body.
So far, researchers have harnessed swimming bacteria for targeted drug delivery. In one case, magnetotactic bacteria guided by an external magnetic field carried nanosize liposomes loaded with a chemotherapy drug to mouse tumors. But bacteria are prime targets for the immune system that are often destroyed before they reach their destination.
See “Bacteria as Living Microrobots to Fight Cancer”
Now, a team at the University of California, San Diego has built a microscopic robot—or microrobot—using Chlamydomonas reinhardtii, a species of microalgae, which are less likely to elicit an immune response than bacteria, study coauthor Liangfang Zhang tells The Scientist.
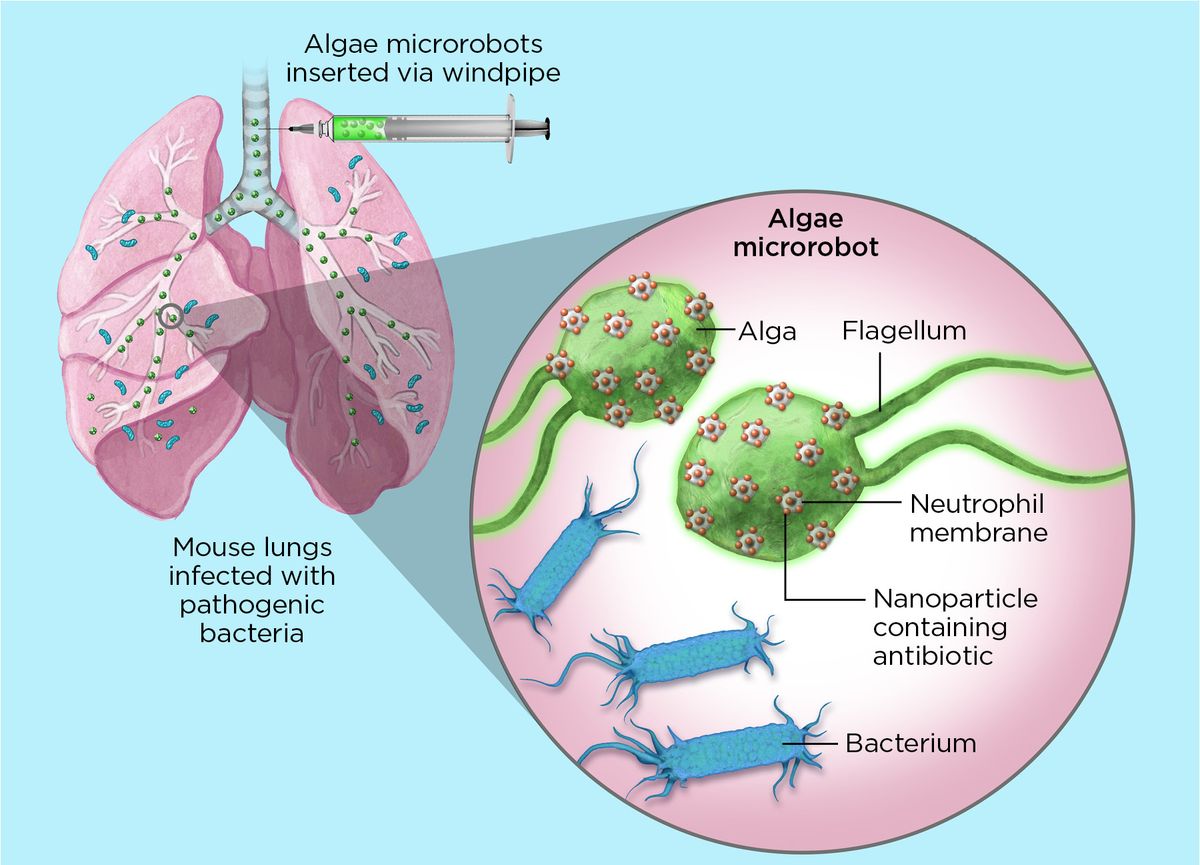
Zhang and his colleagues attached antibiotic-filled nanoparticles to the microbes’ surfaces using click chemistry, the Nobel Prize-winning
method that uses rapid reactions to connect molecules. Inside the body, modified algae beat their flagella to swim through the blood and dive deep into tissues. Each nanoparticle is wrapped in a neutrophil membrane, which promotes immune evasion and allows the microrobots to latch onto pathogens, depositing the drugs in their vicinity.
Treatment with microalgae worked despite a dose of antibiotics 3,000 times smaller than was needed intravenously.
The researchers tested the algae in mice with a severe form of pneumonia caused by Pseudomonas aeruginosa bacteria. Known as ventilator-associated pneumonia (VAP), the potentially fatal infection is picked up by human patients during ventilator use in hospitals. Microrobots were delivered directly into mouse lungs through a tube leading into the windpipe. After one week, infections disappeared in all treated mice. Their untreated littermates died within three days.
The researchers then compared the microrobots to intravenous injection, the current standard treatment for VAP. Treatment with microalgae worked despite a dose of antibiotics 3,000 times smaller than was needed intravenously, which could reduce side effects, says University of Texas at Austin bioengineer Yuebing Zheng, who was not involved in the study.
Taking advantage of the algae’s natural fluorescence, the researchers dissected and imaged the mouse lungs. Light radiated from the whole organ for over 24 hours and from homogenized lung tissue for three days, indicating that the robots had dispersed throughout the tissue and dodged immune attack long enough for successful drug delivery.
Algae microrobots may open “new horizons” in drug targeting, says Zheng, potentially offering an “effective solution” to the problem of low drug absorption.
Finding the best way to get microrobots into the body is still an “ongoing effort,” says Zhang. Algae can be directed into the lungs of ventilated patients already fitted with a breathing tube. But for other diseases, he envisions less invasive methods such as an inhaler that could be administered at home. Unlike typical inhalers, however, these would need to keep organisms alive for the treatment to be effective. Finding a way to make that work won’t be easy, he says.