What Is Quorum Sensing?
Individual bacteria use quorum sensing to detect and respond to changes in cell density as a coordinated group. For example, the first reported evidence of quorum sensing was in two marine bacterial species that emit light in response to high cell density, which is responsible for bioluminescence in various marine hosts. Researchers have identified many species of bacteria that interrelate gene regulation with their neighbors to control a variety of processes, including symbiosis, virulence, antibiotic resistance, and biofilm formation.1

How Does Quorum Sensing Work?
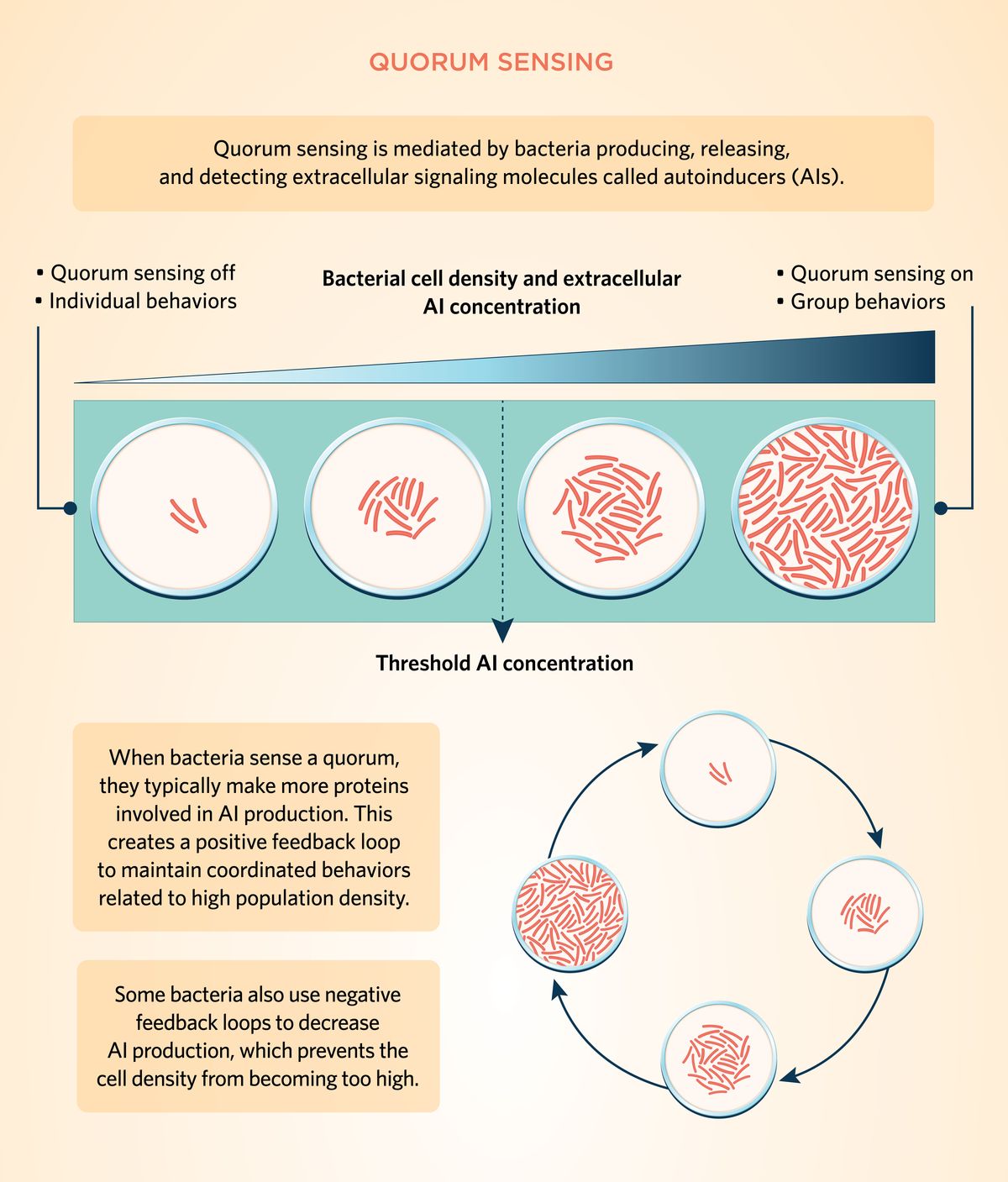
Quorum sensing is mediated by bacteria producing, releasing, and detecting extracellular signaling molecules called autoinducers. Typically, when individual bacteria divide, they produce and release autoinducers. When cell density is low, these molecules diffuse in the environment, and bacteria regulate gene expression independently. Autoinducer levels increase as bacteria continue to divide, and when the extracellular concentration of these signaling molecules reaches a certain threshold, individual bacteria detect that the quorum threshold has been reached. As a result, they regulate signaling pathways and gene transcription in response to the surrounding cell population density. When bacteria sense a quorum, they typically make more proteins involved in autoinducer production, which creates a positive feedback loop to maintain coordinated behaviors related to high population density. Induction of the feedback loop is why quorum signaling molecules are commonly called autoinducers.1-5
What Bacteria Use Quorum Sensing?
Both gram-negative and gram-positive bacteria use quorum sensing. Generally, gram-negative bacteria employ acylated homoserine lactones as autoinducers, which diffuse into the environment passively. In contrast, gram-positive bacteria typically use processed oligopeptides, which must be actively secreted through their cell wall.1,2
What Is Interspecies Quorum Sensing?
Intraspecies quorum sensing allows a variety of bacteria to communicate and alter their behavior in response to bacteria of the same species. Additionally, interspecies quorum sensing between different bacterial species can lead to both conflict and collaboration. For example, enteric bacteria in the human gut microbiome coordinate behavior with each other and against harmful bacteria. Unfortunately, interspecies quorum sensing is not always to the benefit of humans; some bacteria coordinate with one another to enhance each other’s virulence and form symbiotic relationships to create biofilms that protect pathogens from antibiotics.1,2,6
How Do Bacteria Use Quorum Sensing to Control Pathogenicity?
Bacteria regulate virulence genes and other pathogenic factors with quorum sensing. Activating virulence genes at low cell density might alert the host defence response, which prevents invading bacteria from establishing themselves. As such, opportunistic bacteria control virulence genes through quorum sensing to wait until they achieve a sufficient cell density to overpower host defence mechanisms. Once the optimum autoinducer concentration threshold is reached, they synthesize virulence factors to ensure their infection and survival.7
How Do Researchers Apply Quorum Sensing?
The next generation of antibiotics
Bacterial pathogens often develop resistance to antibiotics. As a result, researchers are on the lookout for new therapeutic strategies to treat bacterial infections. Antimicrobials that target quorum sensing pathways provide a promising avenue to prevent inter- and intraspecies communication, without introducing evolutionary pressures to drive resistance. Scientists are investigating species-specific quorum sensing receptor inhibition and quorum quenching to inhibit signaling molecules and attenuate bacterial virulence factors, such as toxin production and biofilm formation.1,7
A viral parallel to quorum sensing
While studying quorum sensing’s role in mediating viral infection of bacteria, researchers identified a comparable communication system in temperate bacterial phages—viruses that enter either the lytic or lysogenic pathway when they infect a bacterium. Viruses in the lytic pathway kill and lyse their host bacterial cell, but in the lysogenic cycle, the phage integrates its genetic material into the bacterial genome and keeps its host alive. Researchers discovered that some phages monitor the concentration of arbitrium—the viral equivalent of an autoinducer—to decide when to switch from the lytic to lysogenic pathway. This decision is based on the density of lysed infected bacteria in their environment, which helps viruses avoid depleting all available hosts. Scientists hypothesized that arbitrium signals may also alter the activity of important bacterial genes, and that this quorum sensing-like communication system may be common among phages and possibly some human viral pathogens.8-10
Synthetic biological circuits
In synthetic biology, scientists design cellular components to perform logical functions that mimic those of electronic circuits. A classic example of a synthetic biological circuit is the lac operon, which is designed based on a naturally-occurring mechanism in E. coli that controls a metabolic switch in gene expression.11,12 Researchers use similar principles to engineer quorum sensing in different systems, including rewiring autoinducers that regulate toxic genes to control population size and growth rate in bacteria, and constructing systems to control population density in mammalian cells. Scientists also study synthetic quorum sensing circuits to find ways to control biofilms or enable drug delivery.13-16
References
- M.B. Miller, B.L. Bassler, “Quorum sensing bacteria,” Annu Rev Microbiol, 55:165-99, 2001.
- W.J. Windsor, “How Quorum Sensing Works,” American Society for Microbiology, https://asm.org/Articles/2020/June/How-Quorum-Sensing-Works, accessed October 19, 2022.
- R.T. Pena, “Relationship between quorum sensing and secretion systems,” Front Microbiol, 10:1100, 2019.
- C.D. Sifri, "Healthcare epidemiology: quorum sensing: bacteria talk sense,” Clin Infect Dis, 47(8):1070-6, 2008.
- K.C. Tu et al., “Negative feedback loops involving small regulatory RNAs precisely control the Vibrio harveyi quorum-sensing response,” Mol Cell, 37(4):567-79, 2010.
- S. Mukherjee, B.L. Bassler, “Bacterial quorum sensing in complex and dynamically changing environments,” Nat Rev Microbiol, 17(6):371-82, 2019.
- S. Haque et al., “Quorum sensing pathways in gram-positive and -negative bacteria: potential of their interruption in abating drug resistance,” J Chemother, 31(4):161-87, 2019.
- Z. Erez et al., “Communication between viruses guides lysis-lysogeny decisions,” Nature, 541(7638):488-93, 2017.
- E. Callaway, “Do you speak virus? Phages caught sending chemical messages,” Nature, https://www.nature.com/articles/nature.2017.21313, accessed October 24, 2022.
- A. Stokar-Avihail et al., “Widespread utilization of peptide communication in phages infecting soil and pathogenic bacteria,” Cell Host Microbe, 25(5):746-55.e5, 2019.
- T.S. Gardner et al., “Construction of a genetic toggle switch in Escherichia coli,” Nature, 403:339-42, 2000.
- H. Kobayashi, et al., "Programmable cells: Interfacing natural and engineered gene networks," PNAS, 101(22):8414-9, 2004.
- L. You et al., "Programmed population control by cell-cell communication and regulated killing," Nature, 428(6985):868-71, 2004.
- S.H. Hong et al., "Synthetic quorum-sensing circuit to control consortial biofilm formation and dispersal in a microfluidic device," Nat Comm, 3(1):613, 2012.
- M.O. Din et al., "Synchronized cycles of bacterial lysis for in vivo delivery," Nature, 536(7614):81-5, 2016.
- Y. Ma et al., "Synthetic mammalian signaling circuits for robust cell population control," Cell, 185(6):967-79.e12, 2022.