What Are Stem Cells?
Stem cells are unspecialized cells capable of self-renewal that can differentiate into other types of cells. They cannot survive outside of their environment without specific factors and cytokines. Stem cells exist both in embryos and adult tissue, and their developmental potential decreases as they become more specialized. For example, a unipotent stem cell cannot differentiate into as many cell types as a pluripotent stem cell.1,2
Mechanisms of Stemness
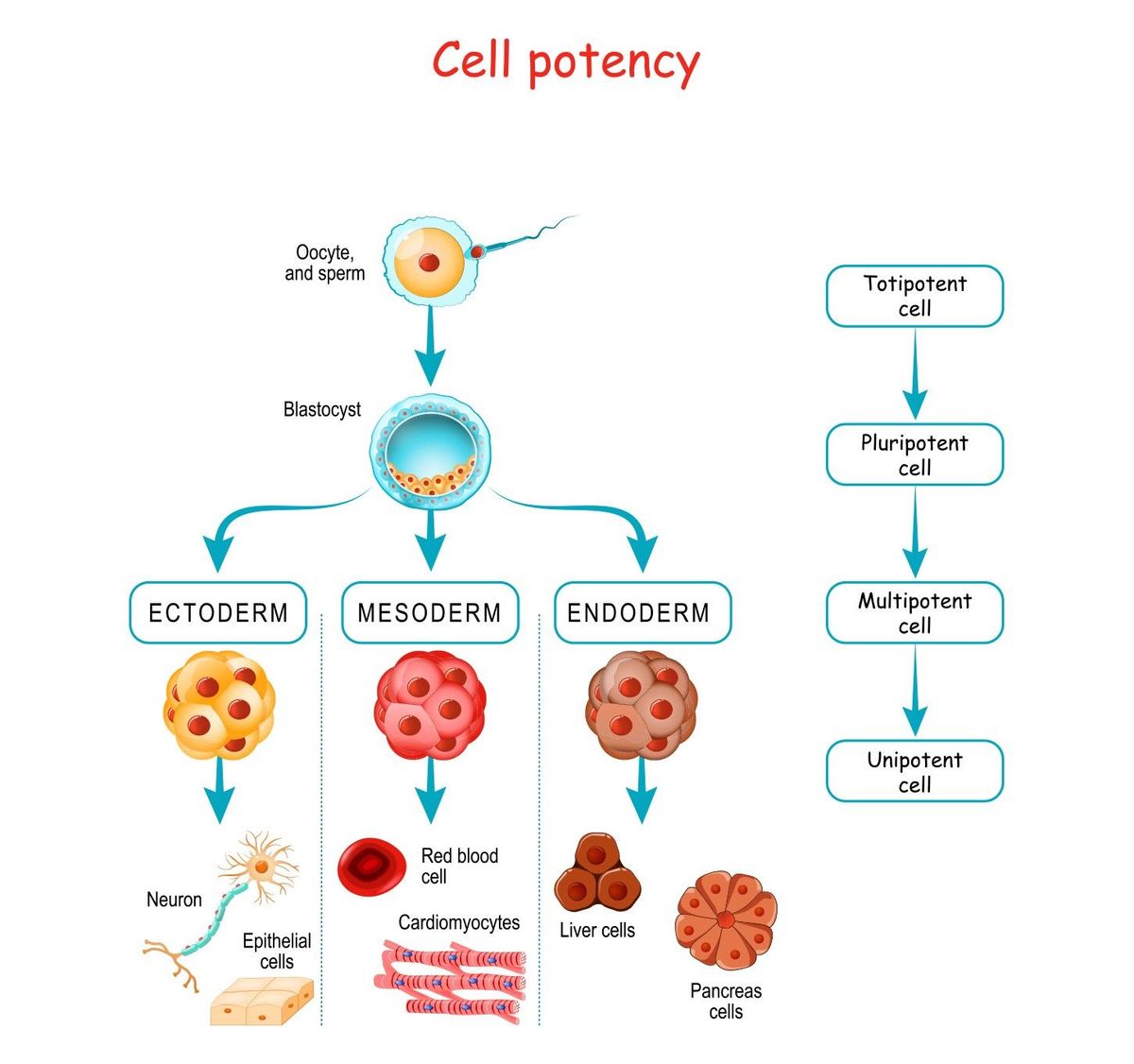
The developmental potential, or potency, of a stem cell varies based on its specialization. Totipotent stem cells have the highest differentiation potential. Unlike pluripotent stem cells, totipotent stem cells can divide and differentiate into every cell that makes a whole organism, including both embryonic and extraembryonic structures. An example of a totipotent cell is a zygote that develops after a sperm fertilizes an egg. As the totipotent zygote divides into more specialized cells, it forms a blastocyst that contains pluripotent cells in the inner cell mass, including embryonic stem cells (ESCs).1,3
Human pluripotent stem cells give rise to all three primary germ layers during embryonic development. This means that they can develop into all cells of the adult body, but do not form extraembryonic structures such as the placenta. During embryogenesis, ESCs differentiate into one of three germ layers: ectoderm, mesoderm, or endoderm. These layers produce differentiated cells and tissues. After embryogenesis, pluripotent stem cells also exist as undifferentiated cells throughout the organism. These cells proliferate to form the next generation of stem cells and differentiate into specialized cells under specific physiological conditions.1,3 Conversely, the stem cell environment and specific stem cell factors can promote the dedifferentiation of specialized cells and return them to a primitive state of development or stemness.2
How Do Researchers Use Pluripotent Stem Cells?
Pluripotent stem cells can self-renew indefinitely while maintaining the ability to differentiate into all cell types in vitro and in vivo. Because of this, researchers use pluripotent stem cell lines to study early development. These cell lines are also a source of unspecialized cells with therapeutic potential. Scientists culture pluripotent stems cells to understand how they may be employed in regenerative medicine, disease modeling, and drug discovery.1,3
Embryonic Development Research
Pluripotent stem cell features evolve as development proceeds through embryogenesis, and different stages of this process are marked by distinct cellular transcriptional and epigenetic signatures. During development, cells mature through a continuum of pluripotent states with unique properties that researchers can capture in vitro with stable pluripotent stem cell types. Additionally, the discovery of iPSCs demonstrated that scientists can change cell fate artificially by activating only a few transcription factors. This finding advanced researchers’ understanding of epigenetic mechanisms that commit specialized cells to their differentiated states during development.3,4
Disease Modeling with Pluripotent Stem Cells
Scientists generate a variety of disease-relevant cell types with human ESCs and iPSCs using differentiation protocols that mimic in vivo organogenesis. Thus, researchers use pluripotent stem cells to model diseases with the aim of developing therapies. For example, iPSC-derived disease-specific stem cells are useful in the study of degenerative disorders, and to gain insight into diseases that lack suitable preclinical models. Scientists also apply pluripotent stem cells in disease modeling with organoids, 3D structures derived from stem cells, progenitor cells, and/or differentiated cells that self-organize to recapitulate aspects of the native tissue structure and function in vitro.4,5
Regenerative Medicine and Drug Discovery with Patient-Specific Pluripotent Stem Cells
Regenerative medicine aims to restore the function of specific tissues for patients with severe injuries or chronic disease conditions. Scientists use disease-specific iPSCs to investigate possible degenerative disorder treatments with stem cell transplants. In addition, patient-specific pluripotent stem cells provide a promising platform for drug discovery and cell therapy in vitro. For example, scientists study patient-derived iPSCs in vitro to investigate the effects of mutations linked to disease risk that are identified in genome-wide association studies (GWAS).4–6
References
- W. Zakrzewski et al., “Stem cells: past, present, and future,” Stem Cell Res Ther, 10:1-22, 2019.
- P.M. Aponte, A. Caicedo, “Stemness in cancer: Stem cells, cancer stem cells, and their microenvironment,” Stem Cells Int, 2017:1-17, 2017.
- S. Morgani et al., “The many faces of pluripotency: in vitro adaptations of a continuum of in vivo states,” BMC Dev Biol, 17:1-20, 2017.
- M. Stadtfeld, K. Hochedlinger, “Induced pluripotency: history, mechanisms, and applications,” Genes Dev, 24:2239-63, 2010.
- W. Hu, M.A. Lazar, “Modelling metabolic diseases and drug response using stem cells and organoids,” Nat Rev Endocrinol, s41574-022-00733-z, online ahead of print, 2022.
- R.S. Mahla, “Stem cells applications in regenerative medicine and disease therapeutics,” Int J Cell Biol, 2016:1-24, 2016.