Stay up to date on the latest science with Brush Up Summaries.
What Are Artificial Organs?
Scientists engineer artificial organs for integration into the human body to replace, duplicate, or augment functional, naturally occurring organs.1 They pose a solution to organ donor shortages, and can also be used as medical training tools.2
Based on the materials researchers use to produce them, artificial organs are divided into three main classes. Mechanical artificial organs are made exclusively of inanimate polymers such as plastics and metals; biomechanical organs involve both living materials such as cells and inanimate materials; and biological or bioartificial organs can be made of living cells and biodegradable polymers.1
How Are Artificial Organs Grown or Manufactured?
In general, organ manufacturing involves architectural predesign, preparation of materials and tools, cell seeding or integration, and tissue maturation. Additionally, tissue or organ engineering requires a scaffold to act as a template for tissue regeneration. Researchers produce scaffolds with techniques such as 3D printing and decellularizing tissue.1,3 For example, scientists may use 3D printing to manufacture tissue scaffolds that mimic the extracellular matrix. They can create hydrogels and seed various cell types, including stem cells, onto the scaffold, which can be incubated in a bioreactor for cell proliferation and maturation. Additionally, researchers are honing 3D bioprinting methods that use cell-encapsulated printing inks called bioink for scaffold generation and seeding. These methods incorporate living cells, along with other biomaterials such as polymers and growth factors prior to printing. 1,2
Decellularized tissue techniques function in a conceptually similar way, but rely on biologically-produced scaffolding. Researchers omit the predesign stage of manufacturing and utilize pre-existing organ architectures that they treat to remove cells while maintaining the extracellular matrix.1,4
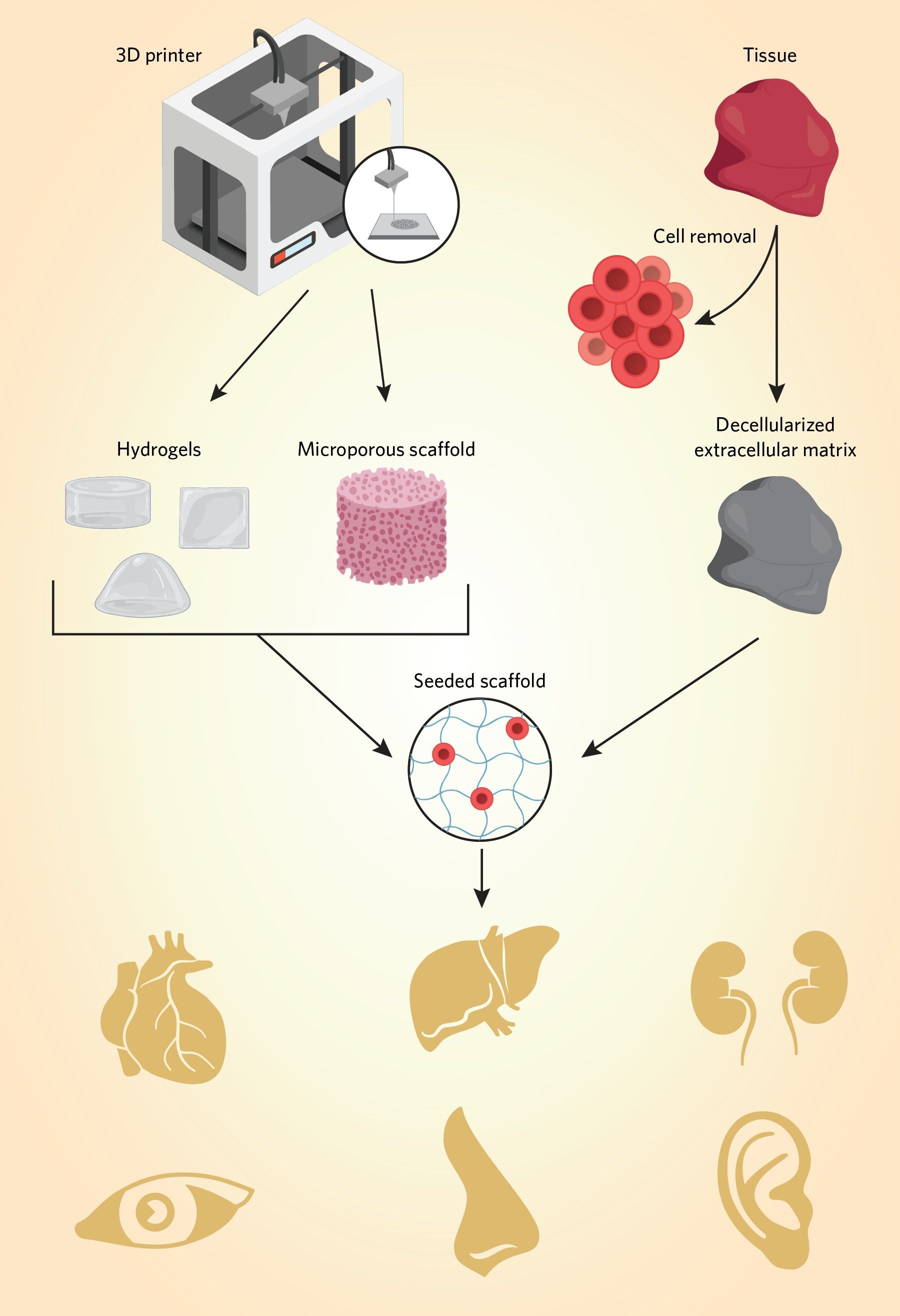
Examples of Artificial Organs and How They Work
Artificial sensory organs
Researchers have long attempted to develop treatment strategies for naturally occurring sensory dysfunctions and injuries of the nervous system. For example, autologous nerve transplantation or nerve grafting has been considered a gold standard reconstruction technique. However, transplanted nerve grafts serve as scaffolds for nerve regeneration, which is a slow biological process. As a result, functional recovery after nerve grafting can be inefficient at replacing or repairing nerve-dependent organ function.5
Artificial sensory organs are a prosthetic means of sending information to the brain without relying on nerve regeneration. They are roughly classified as visual devices for light, auditory devices for sound, olfactory gustatory devices for taste and flavor, and tactile or feeling devices for physical stimulation such as thermal pain sensation. Typically, they function by electrically stimulating nerves, such as the optic or auditory nerves. Recent clinical progress of such devices includes the artificial retina, cochlear implants such as the implantable bone anchored hearing aid (BAHA), and taste and olfactory sensors. Additionally, researchers are developing new types of artificial nerves based on brain–computer interface (BCI) science. One example of this technology is a device that compensates for damaged spinal cord function and controls paralyzed muscle with signals synthesized from the brain and muscle activity of patients.5,6
Artificial kidney and artificial liver
There are many iterations of artificial filtration systems to treat kidney and liver disease. However, external systems such as dialysis machines and other bioartificial supports physically limit patient activity, require adherence to strict dietary and medication regimens, and have high clinical costs.7,8
Conventionally, renal assistance devices (RADs) are extracorporeal artificial systems that rely on a combination of a conventional hemofilter and a bioreactor. They mimic glomerular filtration and drive essential metabolic, endocrinologic, and immunologic kidney functions. Researchers creating artificial organ devices are preclinically investigating methods to miniaturize and implant RADs. In addition to RADs, scientists develop renal scaffolds for bioartificial kidneys from pig kidneys, discarded human kidneys, or polymers such as collagen, hyaluronic acid, alginate, agarose, chitosan, fibrin, and gelatin.7
Similarly, artificial livers may be a solution to organ donor shortages for treating end-stage liver failure and an alternative to extracorporeal artificial supports. For example, researchers made artificial “liver-buds” from human induced pluripotent stem cells (iPSCs) in vitro and transplanted them to successfully rescue an animal model of liver failure.8,9
Artificial heart
Scientists and clinicians can use 3D printing for cardiac surgical planning and creating custom-fit implants. Researchers have also created devices for cardiac tissue maturation that enhance implant viability, such as cardiac-specific extracellular matrices made of animal-derived bioink or decellularized cardiac tissue.10 Additionally, in proof-of-concept studies, researchers have manufactured cardiac structures such as branched coronary arteries and embryonic hearts with hydrogels. Using computed tomography (CT) and magnetic resonance imaging (MRI) data, they printed hydrated materials such as alginate, collagen, and fibrin to build mechanically robust and complex 3D anatomical cardiac architectures.11
References
- X. Wang, “Bioartificial organ manufacturing technologies,” Cell Transplant, 28(1):5-17, 2019.
- A. Dine et al., “A dual nozzle 3D printing system for super soft composite hydrogels,” HardwareX, 9:e00176, 2021.
- X. Wang et al., “A combined rotational mold for manufacturing a functional liver system,” J Bioact Compat Polym, 30(4):1-16, 2015.
- A. Gleadall et al., “Review of additive manufactured tissue engineering scaffolds: relationship between geometry and performance,” Burns Trauma, 6:19, 2018.
- T. Nakamura et al., “Artificial sensory organs: latest progress,” J Artif Organs, 21(1):17-22, 2018.
- T. Ifukube, “Artificial organs: recent progress in artificial hearing and vision,” J Artif Organs, 12(1):8-10, 2009.
- P.R. Corridon et al., “Bioartificial kidneys,” Curr Stem Cell Rep, 3(2):68-76, 2017.
- R. Tandon, S. Froghi, “Artificial liver support systems,” J Gastroenterol Hepatol, 36(5):1164-79, 2021.
- T. Takebe et al., “Vascularized and functional human liver from an iPSC-derived organ bud transplant,” Nature, 499(7459):481-84, 2013.
- V. Sedlakova et al., “3D bioprinted cardiac tissues and devices for tissue maturation,” Cells Tissues Organs, 211(4):406-19, 2021.
- T.J. Hinton et al., “Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels,” Sci Adv, 1(9):e1500758, 2015.