ABOVE: © iStock.com, Artur Plawgo
Remnants of ancient viral DNA are still active in the genomes of animals alive today. At some point in evolutionary history, many of these so-called endogenous retroviruses inserted themselves into the DNA of their host, and their genetic code has been present ever since. Studies have found that this leftover DNA still serves crucial roles unique to mammals.
In a study published in Development on September 26, scientists say they’ve characterized two retrovirus-derived genes that fight infections in the brains of mammals that give birth to live young, a group known as eutherians.
Study coauthor Tomoko Kaneko-Ishino, a geneticist at Tokai University in Japan, says it took her more than 30 years to uncover the function of two virus-derived genes. In an email to The Scientist, she writes that the new work’s roots extend back to a 1989 study on genomic imprinting, a phenomenon where maternal or paternal genes shut down the expression of genes from the other parent. Genomic imprinting is unique to mammals, and Kaneko-Ishino was curious how mammals evolved imprinting-related genes. She hypothesized at the time that the source of these new genes “might be exogenous DNA fragments derived from viruses, and mammals might have evolved to take advantage of them.”
See “Viral Remnants Help Regulate Human Immunity”
Eleven years later, Kaneko-Ishino and her colleagues (as well as other researchers around the same time) discovered two virus-derived imprinted genes, Peg10 and Peg11, involved in placenta formation, another mammalian innovation. Kaneko-Ishino's group later investigated genes with similar sequences called retrotransposon Gag-like (RTL) genes (formerly sushi-ichi retrotransposon homolog, or SIRH, genes).
Over the next decade, Kaneko-Ishino’s team studied the function of RTL genes, and in experiments that would begin the new study, she compared the genomes of animals in various phylogenetic groups and found that two of these genes, RTL5 and RTL6, were evolutionarily conserved among eutherians but not in other mammals. “I thought it must be important,” she writes.
But further experiments suggested otherwise. Knocking out the genes in mice didn’t seem to do much. Mice appeared healthy without the genes, and for 15 years, as she and her team studied the animals, they failed to pinpoint the purpose of the genes or their proteins, despite trying various “gold standard” techniques, Kaneko-Ishino says.
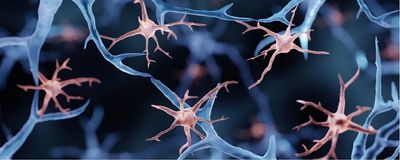
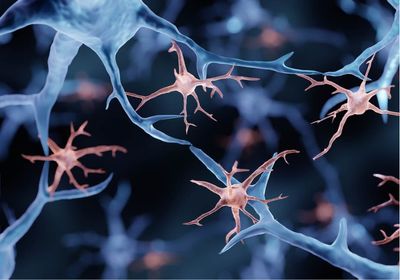
She unearthed another clue three years ago, when her team used genetic engineering to attach fluorescent proteins to the ends of the RTL proteins in mice and saw that RTL5 and RTL6 were localized in immune cells in the brain called microglia. But the signal was very weak.
Kaneko-Ishino and her team began looking for ways to enhance the signal, and, because only immune cells expressed the proteins, they thought that infection might do the trick. They began injecting fluorescently labeled toxins, including the bacterial toxin lipopolysaccharide (LPS) as well as nonmethylated DNA and double-strand RNA, both common viral warning signals, directly into the mice’s brains.
After an LPS injection, the researchers saw a sharp increase in the amount of RTL6 protein in the brain at the injection site. They also found that LPS lingered longer in the brains of RTL6 knockout mice than their normal counterparts, while RTL5 knockout mice were slower at clearing double-strand RNA. The study authors hypothesize that the two RTL proteins might clear out pathogenic substances during infection.
But surprisingly, says Kaneko-Ishino, RTL6 and RTL5 gene expression levels didn’t increase even in the presence of toxins. “They seem to be expressed all the time,” she explains, but the proteins only aggregate in response to infection.
The authors write that this is the first evidence of virally derived, eutherian-specific genes involved in immunity. “It is quite interesting that mammals reuse virus-derived genes not only for [the] innate immune system but also in formation of [the] placenta,” writes Kaneko-Ishino.
Florent Ginhoux, an immunologist at the Singapore Immunology Network who was not involved in the work, says that the study describes “an interesting observation,” but that he would have liked to see the authors test whether these genes are expressed solely in microglia or in other immune cells as well, and see if these genes may give rise to microglia’s unique functions. Microglia and other macrophages differentiate from cells that are conserved among primitive species beyond mammals, he notes, and the use of these genes may not be unique to microglia.
Kaneko-Ishino writes that her group is planning on studying whether RTL5 and RTL6 are microglia-specific in a future experiment. “In [the] normal state, Rtl5 and Rtl6 mRNA expression is observed in other tissues and organs, but we have not seen the RTL5 and RTL6 protein expression other than [in] the brain,” she says, “but I think it is possible it is expressed” in macrophages in other tissues during infections.