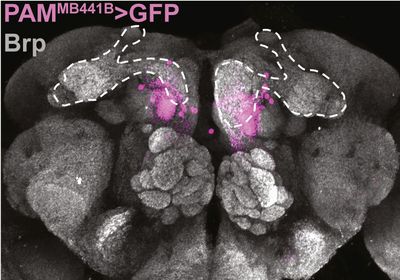
ABOVE: The population of neurons (pink) in the fly brain to which the gut-secreted peptide CCHa1 signals Figure 5C FROM Cell,DOI: 10.1016/j.cell.2023.02.022, 2023
Previous work has pointed to diet—including protein intake—as one potential factor influencing how soundly we sleep. A study published Wednesday (March 22) in Cell adds evidence to the role of food by reporting that a protein-rich diet decreases the arousability of flies and mice. Furthermore, the researchers unraveled the mechanism underlying this effect in flies: dietary proteins activate cells in the gut that secrete a peptide, which signals to a group of neurons responsible for regulating response to mechanical vibrations.
The team wanted “to understand how we disconnect from all the sensory [information] when we’re sleeping,” says coauthor Iris Titos, a neuroscientist at Harvard Medical School. Previous research had pointed to a “strong genetic component,” so the first step in answering their question was to analyze the roles of around 3,400 fly genes. By silencing each of them using RNA interference and assessing how this affected the animals’ arousability, the team found around 160 genes resulting in hypo- or hyperarousable flies—among them, a neuropeptide and its receptor, on which they decided to focus their efforts.
The neuropeptide in question, called CCHa1, is known to be synthesized in both the brain and the gut. When researchers depleted it locally from the brain and gut, they found that its elimination in the gut was sufficient to increase arousability in flies. Since the cells that secrete it in the gut are activated by dietary proteins and amino acids, Titos and her colleagues assessed the role of food in this effect. They found that a diet supplemented with a protein mix increased CCHa1 levels in the flies’ gut and made them less responsive to vibrations while they slept. In contrast, sugar and fat supplementation did not affect either of these measures.
Finally, by searching for the population of neurons with which this peptide communicates, the team’s experiments revealed that CCHa1 is received by a subset of dopaminergic neurons in the brain that modulate responsiveness to vibrations.
Experiments in mice showed that a protein-enriched diet also made these animals more difficult to wake up in response to mechanical vibrations, although it is unclear whether the mechanism is the same as in flies. “It’s a similar phenomenon,” says Harvard Medical School’s Dragana Rogulja, who led the study, “but we don’t know yet if it is the same molecules” that are involved, a question her team is currently interested in addressing.
Throughout the article, Rogulja, Titos, and colleagues describe the effect of protein intake and CCHa1 levels as promoters of “deeper sleep” in these animal models. University of Queensland neuroscientist Bruno van Swinderen, who was not involved in the study, advises caution in the use of this term. Whereas these results show evidence that protein supplementation results in an “increased arousal threshold,” this is not equivalent to deeper sleep or better sleep quality, he emphasizes. He argues that deeper sleep involves specific functions, and within this work, such functions were not assessed. The results, so far, mainly show that the animals become less responsive to stimuli, he says.
See “Waves of Fluid Bathe the Sleeping Brain, Perhaps to Clear Waste”
In a follow-up email to The Scientist, Rogulja writes that “increasing arousal threshold is an important component of sleep regulation in general, and deep sleep requires highest arousal threshold,” adding that “being easily awoken does mean that you are a light sleeper.” She acknowledges that there are indeed “multiple components of deep sleep, but arousal threshold is an essential one.”
Nonetheless, van Swinderen says that in terms of the gut-brain axis connection, this is “an interesting story.” It’s becoming clear that what you eat affects your behavior—in this case, even your arousal threshold, he concludes.