ABOVE: COURTESY OF EMMA KURZ & THE BAR-SAGI LAB AT NYU LANGONE SCHOOL OF MEDICINE; © ISTOCK.COM, BUBAONE; © ISTOCK.COM, LIBRE DE DROIT; © ISTOCK.COM, VITANOVSKI; © ISTOCK.COM, MD ARIFUL ISLAM; © ISTOCK.COM, DR_MICROBE
How Exercise Helps Mice Fight Pancreatic Cancer
Perhaps it’s unsurprising to read that there are health benefits to exercise (in mice). Humans with pancreatic cancer were already known to fare better if they maintained an exercise regiment than those who didn’t, but research published in Cancer Cell this June was the first to identify at least one of the underlying biological mechanisms. In the study, scientists found that mice who ran on appropriately adorable, miniaturized treadmills for 30 minutes per day had higher levels of interleukin-15, a cytokine typically released during exercise. The cytokine then mobilized a subset of cancer-killing immune cells, which were better able to infiltrate and destroy the tumor. In a preliminary human analysis, the scientists found that tissue samples taken from human pancreatic cancer patients who participated in an exercise program contained a higher number of those immune cells than did non-exercising controls. With this mechanism in hand, the authors of the study expressed cautious optimism that exercise could help improve treatments for what’s otherwise a particularly difficult, deadly cancer.

Cancer Cells Break Own DNA to Defend Against Radiation
For all of the myriad cancer-fighting tools in our arsenal, it can be hard to avoid feeling like cancer has a leg up. In this case, a study published in Science this April identifies one seemingly counterintuitive way that tumors can survive radiotherapy and preventg the radiation-induced damage to their DNA from spreading to new generations of cells: They actually induce further DNA damage to themselves, which prevents them from initiating mitosis. The researchers concluded that these self-inflicted DNA breaks serve as a decoy—while the cancer cell is busy repairing its own mess, it also has longer to repair the radiation-induced damage before it continues to multiply. Going forward, the researchers say, it may be able to inhibit this process and make radiotherapy more effective at killing off tumors.
Traversing Narrow Channels Helps Metastatic Cancer Cells Survive
Typically, if a cell gets squeezed too hard, it dies. But for a metastasizing cancer cell, the process of squeezing through the narrow channels of the circulatory system may trigger a series of mutations that help the cell stave off programmed cell death while also evading the immune system, according to in vitro and mouse research published in eLife in March. In the experiment, cell nuclei burst open as the cells squeezed their way through a narrow channel, rendering themselves susceptible to various mutations. In general, this suite of changes helped the migrating cancer survive metastasis, which is an otherwise risky journey for a cell to take. However, it’s not yet clear whether this same process occurs in humans, as the mouse model lacked an immune response and the artificial channels in vitro were imperfect models of human blood vessels.

Cancer Cells Gather Speed in Thicker Fluids
This past year, a number of researchers focused their efforts on understanding how cancer cells interact with their surrounding environment. One finding that resulted from this was the discovery, described in Nature this November, that metastasizing cancer cells travel faster through fluids with a viscosity more closely resembling that of bodily fluids than they do through less viscous water. In the study, scientists found that cancer cells can restructure their actin architecture in such a way that they’re better able to plow through thicker fluids, taking in water from their front and blasting it out their rear like a jet. Study coauthor Miguel Valverde, of Pompeu Fabra University in Spain, tells The Scientist that targeting the pathway responsible for these changes—specifically the ion channel TRPV4—could potentially lead to new ways to keep tumors from successfully metastasizing.

Oral Cancer Survives Starvation with Help from Nearby Nerves
How does cancer, notoriously ravenous and endlessly feasting on glucose, survive after it depletes its local microenvironment of fuel? In the case of oral squamous cell carcinoma, a cancer that affects the lining of the mouth, tongue, gums, and lips, the hungry tumor orders delivery from a network of nearby nerves, according to research published in Cell Metabolism this November. In exchange for neural growth factor, which the tumors secrete to promote the growth of pain-sensing nerves closer to them, the nerves release a compound called that induces a cancer survival mechanism called cytoprotective autophagy. This makes the cancer better able to stave off cell death and survive starvation conditions, and also bolsters their resistance to chemotherapy drugs and radiotherapy. “We think [this] finding may inform a kind of new treatment strategy for cancer patients,” study coauthor Ji Tong, an oncologist and oral disease researcher at Shanghai Jiao Tong University School of Medicine, tells The Scientist.
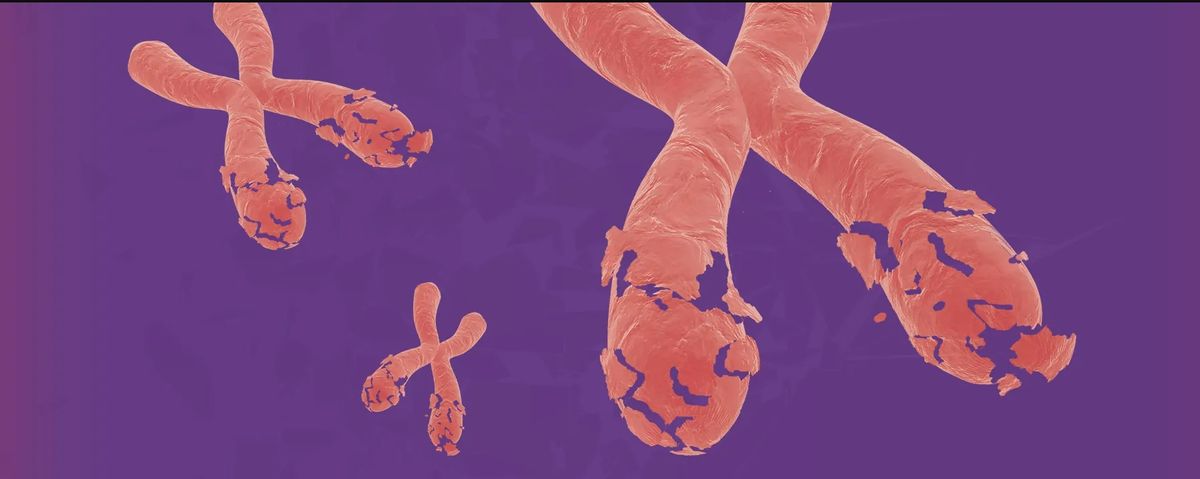
How Chaos in Chromosomes Helps Drive Cancer Spread
In this feature article, Samuel Bakhoum, a cancer biologist at Memorial Sloan Kettering Cancer Center in New York who cofounded the cancer therapeutics company Volastra Therapeutics, reports on how large-scale structural changes to a cell’s chromosomes, can help drive cancer progression, regardless of any specific mutations or genetic changes that a cancer cell may have acquired. Describing myriad studies, including some of his own research, Bakhoum describes how progressing chromosomal instability, stemming from a constant shuffling, breaking, or fusing of chromosomes during cell replication, results in high levels of heterogeneity within a given tumor that makes targeted treatments less effective. “Identifying targetable pathways linked to chromosomal instability is exciting, as it creates an opportunity to therapeutically intervene to tackle a feature of cancer that was hitherto considered undruggable,” he writes.